Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers
- PMID: 26981247
- PMCID: PMC4791966
- DOI: 10.1186/s40425-016-0121-5
Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers
Abstract
Background: We conducted a phase I study with a granulocyte macrophage colony stimulating factor (GMCSF)-expressing oncolytic adenovirus, ONCOS-102, in patients with solid tumors refractory to available treatments. The objectives of the study were to determine the optimal dose for further use and to assess the safety, tolerability and adverse event (AE) profile of ONCOS-102. Further, the response rate and overall survival were evaluated as well as preliminary evidence of disease control. As an exploratory endpoint, the effect of ONCOS 102 on biological correlates was examined.
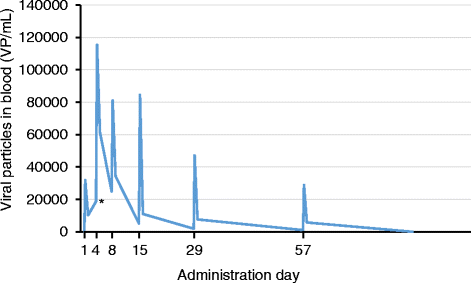
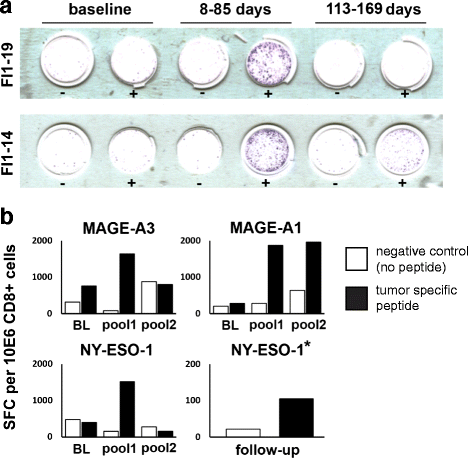
Methods: The study was conducted using a classic 3 + 3 dose escalation study design involving 12 patients. Patients were repeatedly treated intratumorally with ONCOS-102 plus daily low-dose oral cyclophosphamide (CPO). Tumor response was evaluated with diagnostic positron emission tomography (PET) and computed tomography (CT). Tumor biopsies were collected at baseline and after treatment initiation for analysis of immunological correlates. Peripheral blood mononuclear cells (PBMCs) were collected at baseline and during the study to assess antigen specificity of CD8+ T cells by interferon gamma (IFNγ) enzyme linked immunospot assay (ELISPOT).
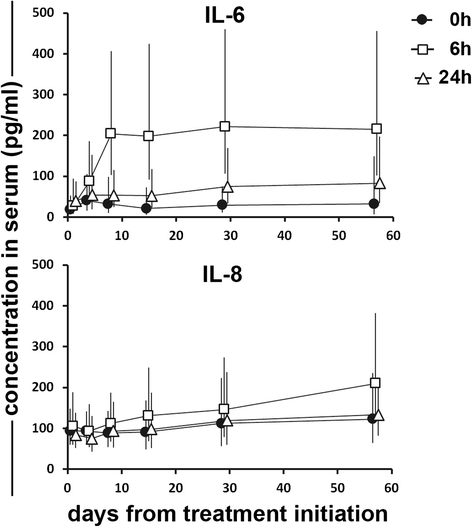
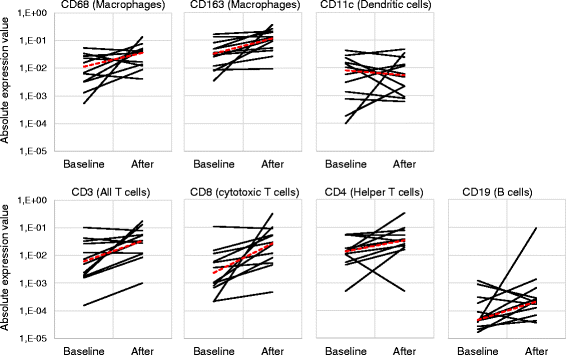
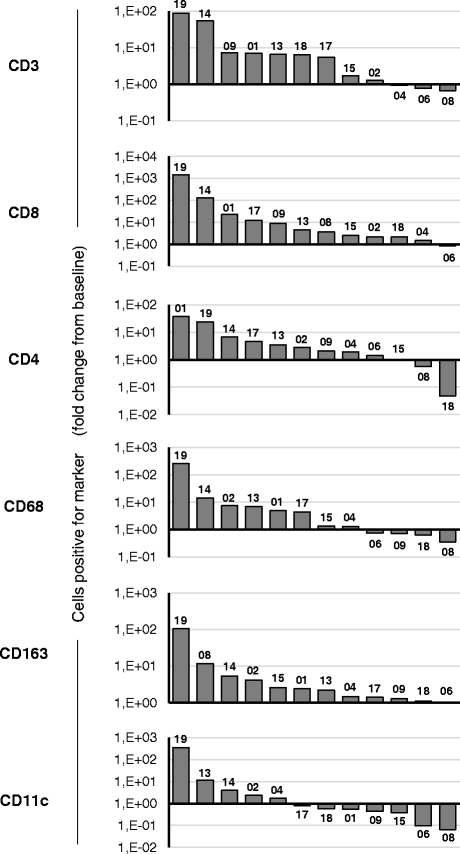
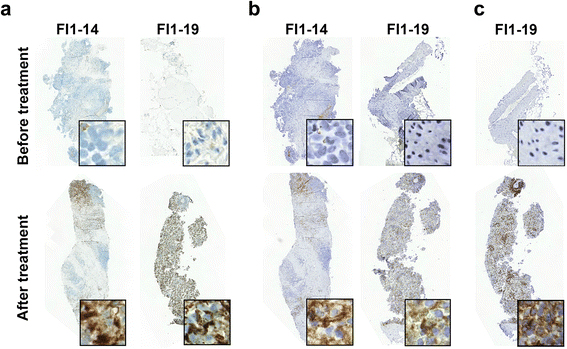
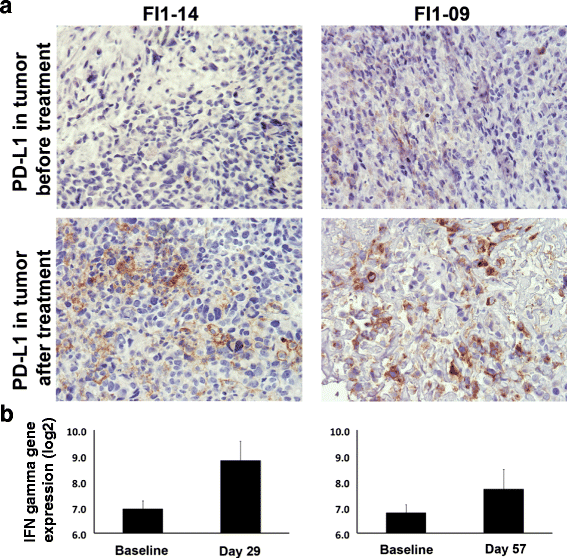
Results: No dose limiting toxicity (DLT) or maximum tolerated dose (MTD) was identified for ONCOS-102. Four out of ten (40 %) evaluable patients had disease control based on PET/CT scan at 3 months and median overall survival was 9.3 months. A short-term increase in systemic pro-inflammatory cytokines and a prominent infiltration of TILs to tumors was seen post-treatment in 11 out of 12 patients. Two patients showed marked infiltration of CD8+ T cells to tumors and concomitant systemic induction of tumor-specific CD8+ T cells. Interestingly, high expression levels of genes associated with activated TH1 cells and TH1 type immune profile were observed in the post-treatment biopsies of these two patients.
Conclusions: ONCOS-102 is safe and well tolerated at the tested doses. All three examined doses may be used in further development. There was evidence of antitumor immunity and signals of clinical efficacy. Importantly, treatment resulted in infiltration of CD8+ T cells to tumors and up-regulation of PD-L1, highlighting the potential of ONCOS-102 as an immunosensitizing agent for combinatory therapies with checkpoint inhibitors.
Trial registration: NCT01598129. Registered 19/04/2012.
Keywords: Anti-tumor immunity; Cytotoxic CD8+ T cell; Immunotherapy; Intratumoral; Oncolytic adenovirus; in situ vaccine.
Figures


References
-
- Greig SL. Talimogene laherparepvec: first global approval. Drugs. 2015 - PubMed
-
- Ranki T, Joensuu T, Jager E, Karbach J, Wahle C, Kairemo K, et al. Local treatment of a pleural mesothelioma tumor with ONCOS-102 induces a systemic antitumor CD8 T-cell response, prominent infiltration of CD8 lymphocytes and Th1 type polarization. Oncoimmunology. 2014;3(10):e958937. doi: 10.4161/21624011.2014.958937. - DOI - PMC - PubMed
-
- Vassilev L, Ranki T, Joensuu T, Jager E, Karbach J, Wahle C, et al. Repeated intratumoral administration of ONCOS-102 leads to systemic antitumor CD8 T-cell response and robust cellular and transcriptional immune activation at tumor site in a patient with ovarian cancer. Oncoimmunology. 2015;4(7):e1017702. doi: 10.1080/2162402X.2015.1017702. - DOI - PMC - PubMed
-
- Fridman WH, Galon J, Dieu-Nosjean MC, Cremer I, Fisson S, Damotte D, et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol. 2011;344:1–24. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
