VEGF: From Discovery to Therapy: The Champalimaud Award Lecture
- PMID: 26981331
- PMCID: PMC4790434
- DOI: 10.1167/tvst.5.2.9
VEGF: From Discovery to Therapy: The Champalimaud Award Lecture
Abstract
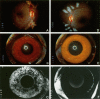
Purpose: Intraocular vascular diseases are leading causes of adult vision loss, and in the mid-1900s, I. C. Michaelson postulated that the retina releases a soluble, diffusible factor that causes abnormal vascular growth and leakage. What became known as "Factor X" eluded investigators for decades.
Methods: The field of cancer research, where Judah Folkman pioneered the concept of angiogenesis, provided the inspiration for the work honored by the 2014 Champalimaud Vision Award. Recognizing that tumors recruit their own blood supply to achieve critical mass, Dr Folkman proposed that angiogenic factors could be therapeutic targets in cancer. Napoleone Ferrara identified vascular endothelial growth factor (VEGF) as such an angiogenic agent: stimulated by hypoxic tumor tissue, secreted, and able to induce neovascularization. VEGF also was a candidate for Factor X, and the 2014 Champalimaud Laureates and colleagues worked individually and collaboratively to identify the role of VEGF in ocular disease.
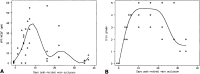
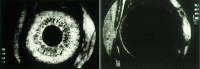
Results: The Champalimaud Laureates correlated VEGF with ocular neovascularization in animal models and in patients. Moreover, they showed that VEGF not only was sufficient, but it also was required to induce neovascularization in normal animal eyes, as VEGF inhibition abolished ocular neovascularization in key animal models.
Conclusions: The identification of VEGF as Factor X altered the therapeutic paradigms for age-related macular degeneration (AMD), diabetic retinopathy, retinal vein occlusion, and other retinal disorders.
Translational relevance: The translation of VEGF from discovery to therapy resulted in the most successful applications of antiangiogenic therapy to date. Annually, over one million patients with eye disease are treated with anti-VEGF agents.
Keywords: António Champalimaud Vision Award; Factor X; age-related macular degeneration (AMD); angiogenesis; vascular endothelial growth factor (VEGF).
Figures










References
-
- Senger DR,, Galli SJ,, Dvorak AM,, Perruzzi CA,, Harvey VS,, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983; 219: 983–985. - PubMed
-
- Leung DW,, Cachianes G,, Kuang WJ,, Goeddel DV,, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989; 246: 1306–1309. - PubMed
-
- Michaelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc U K. 1948; 68: 137–180.
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971; 285: 1182–1186. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

