Pharmacokinetic and pharmacodynamic responses in adult patients with Chagas disease treated with a new formulation of benznidazole
- PMID: 26982179
- PMCID: PMC4804505
- DOI: 10.1590/0074-02760150401
Pharmacokinetic and pharmacodynamic responses in adult patients with Chagas disease treated with a new formulation of benznidazole
Abstract
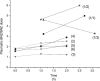
Pharmacological treatment of Chagas disease with benznidazole (BNZ) is effective in children in all stages, but it is controversial in chronically infected adults. We report the pharmacokinetics and pharmacodynamics in six adult patients with Chagas disease treated with the new BNZ formulation (ABARAX®) in doses between 2.5-5.5 mg/Kg/day. All but one patient had plasmatic BNZ concentrations within the expected range. All patients finalised treatment with nondetectable Trypanosoma cruzi quantitative polymerase chain reaction, which remained nondetectable at the six month follow-up. Our data suggests parasitological responses with the new BNZ and supports the hypothesis that treatment protocols with lower BNZ doses may be effective.
Figures
References
-
- Altcheh J, Moscatelli G, Moroni S, Garcia-Bournissen F, Freilij H. Adverse events after the use of benznidazole in infants and children with Chagas disease. Pediatrics. 2011;127:212–218. - PubMed
-
- ANMAT - Administración Nacional de Medicamentos, Alimentos y Tecnología Médica Disposición 5855-12. 2012 anmat.gov.ar/boletin_anmat/octubre_2012/Dispo_5855-12.pdf.
-
- Castro JA, Mecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas disease (American trypanosomiasis) Hum Exp Toxicol. 2006;25:471–479. - PubMed
-
- Andrade AL, Zicker F, Oliveira RM, Silva SA, Luquetti A, Travassos LR, Almeida IC, Andrade SS, Andrade JG, Martelli CM. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–1413. - PubMed
-
- Hasslocher-Moreno AM, do Brasil PE, Sousa AS, Xavier SS, Chambela MC, Silva GMS. Safety of benznidazole use in the treatment of chronic Chagas disease. J Antimicrob Chemother. 2012;67:1261–1266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

