Structural Abnormalities in the Hair of a Patient with a Novel Ribosomopathy
- PMID: 26982655
- PMCID: PMC4794122
- DOI: 10.1371/journal.pone.0149619
Structural Abnormalities in the Hair of a Patient with a Novel Ribosomopathy
Abstract
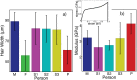
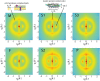
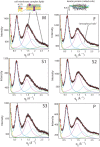
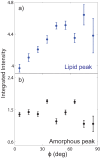
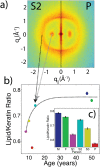
We report the biophysical characterization of hair from a patient with a de novo ribosomopathy. The patient was diagnosed with a mutation on gene RPS23, which codes for a protein which comprises part of the 40S subunit of the ribosome. The patient presents with a number of phenotypes, including hypotonia, autism, extra teeth, elastic skin, and thin/brittle hair. We combined optical microscopy, tensile tests, and X-ray diffraction experiments on hair samples obtained from the scalp of the patient to a multi-scale characterization of the hair from macroscopic to molecular length scales and observe distinct differences in the biophysical properties in the patient's hair when compared to hair from other family members. While no differences were observed in the coiled-coil structure of the keratin proteins or the structure of the intermediate filaments, the patient's hair was 22% thinner, while the Young's modulus remained roughly constant. The X-ray diffraction results give evidence that the amount of lipids in the cell membrane complex is reduced by 20%, which well accounts for the other observations. The pathologies characterized by these techniques may be used to inform the diagnosis of similar de novo mutations in the future.
Conflict of interest statement
Figures





References
-
- Soden SE, Saunders CJ, Willig LK, Farrow EG, Smith LD, Petrikin JE, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Science translational medicine. 2014;6(265):265ra168–265ra168. 10.1126/scitranslmed.3010076 - DOI - PMC - PubMed
-
- Robbins CR. Chemical and Physical Behavior of Human Hair. 5th ed Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. Available from: http://link.springer.com/10.1007/978-3-642-25611-0 - DOI
-
- Astbury W, Sisson WA. X-ray studies of the structure of hair, wool, and related fibres. III. The configuration of the keratin molecule and its orientation in the biological cell. Proceedings of the Royal Society of London Series A, Mathematical and Physical Sciences. 1935;150(871):533–551. 10.1098/rspa.1935.0121 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

