Occupational exposure limit for silver nanoparticles: considerations on the derivation of a general health-based value
- PMID: 26982810
- PMCID: PMC12091348
- DOI: 10.3109/17435390.2016.1148793
Occupational exposure limit for silver nanoparticles: considerations on the derivation of a general health-based value
Abstract
With the increased production and widespread commercial use of silver nanoparticles (AgNPs), human and environmental exposures to silver nanoparticles are inevitably increasing. In particular, persons manufacturing and handling silver nanoparticles and silver nanoparticle containing products are at risk of exposure, potentially resulting in health hazards. While silver dusts, consisting of micro-sized particles and soluble compounds have established occupational exposure limits (OELs), silver nanoparticles exhibit different physicochemical properties from bulk materials. Therefore, we assessed silver nanoparticle exposure and related health hazards in order to determine whether an additional OEL may be needed. Dosimetric evaluations in our study identified the liver as the most sensitive target organ following inhalation exposure, and as such serves as the critical target organ for setting an occupational exposure standard for airborne silver nanoparticles. This study proposes an OEL of 0.19 μg/m(3) for silver nanoparticles derived from benchmark concentrations (BMCs) from subchronic rat inhalation toxicity assessments and the human equivalent concentration (HEC) with kinetic considerations and additional uncertainty factors. It is anticipated that this level will protect workers from potential health hazards, including lung, liver, and skin damage.
Keywords: Clearance; dosimetry; occupational exposure; silver nanoparticles; subchronic inhalation.
Figures


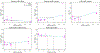
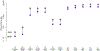
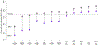
References
-
- ACGIH (American Conference of Governmental Industrial Hygienists).(2013. TLVs and BEIs. ACGIH, Cincinnati,.
-
- Allen BC, Kavlock RJ, Kimmel CA, Faustman EM. 1994. Dose-response assessment for developmental toxicity. II. Comparison of generic benchmark dose estimates with no observed adverse effect levels. Fundamental and applied toxicology 23(4): 487–495. - PubMed
-
- Aschberger K, Johnston HJ, Stone V, Aitken RJ, Hankin SM, Peters SA, et al. 2010. Review of carbon nanotubes toxicity and exposure--appraisal of human health risk assessment based on open literature. Crit Rev Toxicol 40(9): 759–790. - PubMed
-
- Asgharian B, Price O, Miller F, Subramaniam R, Cassee FR, Freijer J, et al. 2009. Multiple-Path Particle Dosimetry Model (MPPD v2.11): A Model for Human and Rat Airway Particle Dosimetry. In Hamner Institutes for Health Sciences Applied Research Associates (ARA), National Institute for Public Health and the Environment (RIVM), and Ministry of Housing, Spatial Planning and the Environment, Editor. Raleigh, North Carolina, USA: Applied Research Associates (ARA).
-
- AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. 2009. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3(2): 279–290. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
