Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model
- PMID: 26983696
- PMCID: PMC4794799
- DOI: 10.1038/srep23076
Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model
Abstract
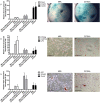
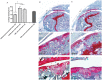
Controversy remains whether articular cartilage has an endogenous stem/progenitor cell population, since its poor healing capacity after injury can lead to diseases such as osteoarthritis. In the joint environment there are mesenchymal stem/progenitor cells (MSCs) in the synovial membrane and synovial fluid that can differentiate into cartilage, but it is still under debate if these cells contribute to cartilage repair in vivo. In this study, we isolated a Sca-1 positive, chondrogenesis capable population of mouse synovial MSCs from C57BL6 and MRL/MpJ "super-healer" strains. Intra-articular injection of Sca-1 + GFP + synovial cells from C57BL6 or MRL/MpJ into C57BL6 mice following cartilage injury led to increased cartilage repair by 4 weeks after injury. GFP expression was detected in the injury site at 2 weeks, but not 4 weeks after injury. These results suggest that synovial stem/progenitor cells, regardless of strain background, have beneficial effects when injected into an injured joint. MSCs derived from MRL/MpJ mice did not promote an increased repair capacity compared to MSCs derived from non-healing C57BL6 controls; however, MRL/MpJ MSCs were observed within the defect area at the time points examined, while C57BL6 MSCs were not.
Figures






References
-
- Pittenger M. F. et al. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 284, 143–147 (1999). - PubMed
-
- De Bari C., De Accio F. D., Tylzanowski P. & Luyten F. P. Multipotent Mesenchymal Stem Cells From Adult Human Synovial Membrane. Arthritis Rheum. 44, 1928–1942 (2001). - PubMed
-
- Dennis E., Bruder S. P., Lennon D. P. & Caplan A. I. In Vitro Differentiation of Bone and Hypertrophic Cartilage from Periosteal-Derived Cells. Exp Cell Res. 195, 492–503 (1991). - PubMed
-
- Johnson K. et al. A Stem Cell–Based Approach to Cartilage Repair. Science. 336, 717–721 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

