Melittin, the Major Pain-Producing Substance of Bee Venom
- PMID: 26983715
- PMCID: PMC5563768
- DOI: 10.1007/s12264-016-0024-y
Melittin, the Major Pain-Producing Substance of Bee Venom
Abstract
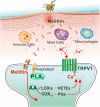
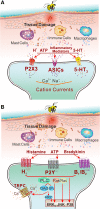
Melittin is a basic 26-amino-acid polypeptide that constitutes 40-60% of dry honeybee (Apis mellifera) venom. Although much is known about its strong surface activity on lipid membranes, less is known about its pain-producing effects in the nervous system. In this review, we provide lines of accumulating evidence to support the hypothesis that melittin is the major pain-producing substance of bee venom. At the psychophysical and behavioral levels, subcutaneous injection of melittin causes tonic pain sensation and pain-related behaviors in both humans and animals. At the cellular level, melittin activates primary nociceptor cells through direct and indirect effects. On one hand, melittin can selectively open thermal nociceptor transient receptor potential vanilloid receptor channels via phospholipase A2-lipoxygenase/cyclooxygenase metabolites, leading to depolarization of primary nociceptor cells. On the other hand, algogens and inflammatory/pro-inflammatory mediators released from the tissue matrix by melittin's pore-forming effects can activate primary nociceptor cells through both ligand-gated receptor channels and the G-protein-coupled receptor-mediated opening of transient receptor potential canonical channels. Moreover, subcutaneous melittin up-regulates Nav1.8 and Nav1.9 subunits, resulting in the enhancement of tetrodotoxin-resistant Na(+) currents and the generation of long-term action potential firing. These nociceptive responses in the periphery finally activate and sensitize the spinal dorsal horn pain-signaling neurons, resulting in spontaneous nociceptive paw flinches and pain hypersensitivity to thermal and mechanical stimuli. Taken together, it is concluded that melittin is the major pain-producing substance of bee venom, by which peripheral persistent pain and hyperalgesia (or allodynia), primary nociceptive neuronal sensitization, and CNS synaptic plasticity (or metaplasticity) can be readily induced and the molecular and cellular mechanisms underlying naturally-occurring venomous biotoxins can be experimentally unraveled.
Keywords: Algogen; Melittin; Nociceptor; Pain; Spinal dorsal horn.
Figures
References
-
- Chen J, Guan SM. Bee venom and pain. In Toxinology: Toxins and Drug Discovery. Edited by Gopalakrishnakone P. New York: Springer; in press.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

