Ciliary Extracellular Vesicles: Txt Msg Organelles
- PMID: 26983828
- PMCID: PMC4886304
- DOI: 10.1007/s10571-016-0345-4
Ciliary Extracellular Vesicles: Txt Msg Organelles
Abstract
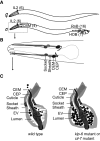
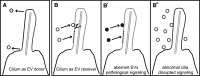
Cilia are sensory organelles that protrude from cell surfaces to monitor the surrounding environment. In addition to its role as sensory receiver, the cilium also releases extracellular vesicles (EVs). The release of sub-micron sized EVs is a conserved form of intercellular communication used by all three kingdoms of life. These extracellular organelles play important roles in both short and long range signaling between donor and target cells and may coordinate systemic responses within an organism in normal and diseased states. EV shedding from ciliated cells and EV-cilia interactions are evolutionarily conserved phenomena, yet remarkably little is known about the relationship between the cilia and EVs and the fundamental biology of EVs. Studies in the model organisms Chlamydomonas and Caenorhabditis elegans have begun to shed light on ciliary EVs. Chlamydomonas EVs are shed from tips of flagella and are bioactive. Caenorhabditis elegans EVs are shed and released by ciliated sensory neurons in an intraflagellar transport-dependent manner. Caenorhabditis elegans EVs play a role in modulating animal-to-animal communication, and this EV bioactivity is dependent on EV cargo content. Some ciliary pathologies, or ciliopathies, are associated with abnormal EV shedding or with abnormal cilia-EV interactions. Until the 21st century, both cilia and EVs were ignored as vestigial or cellular junk. As research interest in these two organelles continues to gain momentum, we envision a new field of cell biology emerging. Here, we propose that the cilium is a dedicated organelle for EV biogenesis and EV reception. We will also discuss possible mechanisms by which EVs exert bioactivity and explain how what is learned in model organisms regarding EV biogenesis and function may provide insight to human ciliopathies.
Keywords: C. elegans; Cilia; Ectosome; Exosome; Extracellular vesicles; Microvesicle.
Figures
Comment in
-
Correspondence.Retina. 2016 Aug;36(8):e78-9. doi: 10.1097/IAE.0000000000001160. Retina. 2016. PMID: 27388737 No abstract available.
References
-
- Bae YK, Qin H, Knobel KM, Hu J, Rosenbaum JL, Barr MM (2006) General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development 133:3859–3870 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

