Understanding the Role of ECM Protein Composition and Geometric Micropatterning for Engineering Human Skeletal Muscle
- PMID: 26983843
- PMCID: PMC4880540
- DOI: 10.1007/s10439-016-1592-8
Understanding the Role of ECM Protein Composition and Geometric Micropatterning for Engineering Human Skeletal Muscle
Abstract
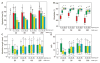
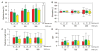
Skeletal muscle lost through trauma or disease has proven difficult to regenerate due to the challenge of differentiating human myoblasts into aligned, contractile tissue. To address this, we investigated microenvironmental cues that drive myoblast differentiation into aligned myotubes for potential applications in skeletal muscle repair, organ-on-chip disease models and actuators for soft robotics. We used a 2D in vitro system to systematically evaluate the role of extracellular matrix (ECM) protein composition and geometric patterning for controlling the formation of highly aligned myotubes. Specifically, we analyzed myotubes differentiated from murine C2C12 cells and human skeletal muscle derived cells (SkMDCs) on micropatterned lines of laminin compared to fibronectin, collagen type I, and collagen type IV. Results showed that laminin supported significantly greater myotube formation from both cells types, resulting in greater than twofold increase in myotube area on these surfaces compared to the other ECM proteins. Species specific differences revealed that human SkMDCs uniaxially aligned over a wide range of micropatterned line dimensions, while C2C12s required specific line widths and spacings to do the same. Future work will incorporate these results to engineer aligned human skeletal muscle tissue in 2D for in vitro applications in disease modeling, drug discovery and toxicity screening.
Keywords: Extracellular matrix; Microcontact printing; Skeletal muscle; Tissue engineering.
Figures






References
-
- Altomare L, Riehle M, Gadegaard N, Tanzi M, Fare S. Microcontact printing of fibronectin on a biodegradable polymeric surface for skeletal muscle cell orientation. International Journal of Artificial Organs. 2010;33:535–543. - PubMed
-
- Bajaj P, Reddy B, Jr, Millet L, Wei C, Zorlutuna P, Bao G, Bashir R. Patterning the differentiation of C2C12 skeletal myoblasts. Integr Biol (Camb) 3:897–909. - PubMed
-
- Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol. 2009;296:C1338–1345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

