Chronic granulomatous disease
- PMID: 26983962
- PMCID: PMC5127417
- DOI: 10.1093/bmb/ldw009
Chronic granulomatous disease
Abstract
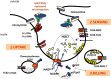
Introduction: Chronic granulomatous disease (CGD) is a primary immunodeficiency characterized by recurrent, life-threatening bacterial and fungal infections of the skin, the airways, the lymph nodes, the liver, the brain and the bones. Frequently found pathogens are Staphylococcus aureus, Aspergillus species, Klebsiella species, Burkholderia cepacia, Serratia marcescens and Salmonella species.
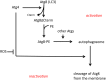
Sources of data: CGD is a rare (∼1:250 000 individuals) disease caused by mutations in any one of the five components of the NADPH oxidase in phagocytic leucocytes. This enzyme generates superoxide and is essential for intracellular killing of pathogens by phagocytes.
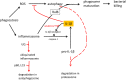
Areas of agreement: CGD patients suffer not only from life-threatening infections, but also from excessive inflammatory reactions.
Areas of controversy: Neither the cause of these inflammatory reactions nor the way to treat them is clear.
Areas timely for developing research: Patient selection for and timing of bone marrow transplantation along with gene therapy.
Keywords: IL-1β; NADPH oxidase; autophagy; autoreactive T cells; chronic granulomatous disease; hyperinflammation.
© The Author 2016. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Roos D, Holland SM, Kuijpers TW. Chronic granulomatous disease. In: Ochs HD, Smith CIE, Puck JM (eds). Primary Immunodeficiency Diseases, A Molecular and Genetic Approach 3rd ed New York: Oxford University Press, 2014,689–722.
-
- Marciano BE, Rosenzweig SD, Kleiner DE et al. . Gastrointestinal involvement in chronic granulomatous disease. Pediatrics 2004;114:462–8. - PubMed
-
- Magnani A, Brosselin P, Beauté J et al. . Inflammatory manifestations in a single-center cohort of patients with chronic granulomatous disease. J Allergy Clin Immunol 2014;134:655–62. - PubMed
-
- Sillevis Smitt JH, Bos JD, Weening RS et al. . Discoid lupus erythematosus-like skin changes in patients with autosomal recessive chronic granulomatous disease. Arch Dermatol 1990;126:1656–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

