Design and synthesis of the superionic conductor Na10SnP2S12
- PMID: 26984102
- PMCID: PMC4800433
- DOI: 10.1038/ncomms11009
Design and synthesis of the superionic conductor Na10SnP2S12
Abstract
Sodium-ion batteries are emerging as candidates for large-scale energy storage due to their low cost and the wide variety of cathode materials available. As battery size and adoption in critical applications increases, safety concerns are resurfacing due to the inherent flammability of organic electrolytes currently in use in both lithium and sodium battery chemistries. Development of solid-state batteries with ionic electrolytes eliminates this concern, while also allowing novel device architectures and potentially improving cycle life. Here we report the computation-assisted discovery and synthesis of a high-performance solid-state electrolyte material: Na10SnP2S12, with room temperature ionic conductivity of 0.4 mS cm(-1) rivalling the conductivity of the best sodium sulfide solid electrolytes to date. We also computationally investigate the variants of this compound where tin is substituted by germanium or silicon and find that the latter may achieve even higher conductivity.
Figures
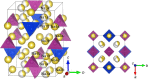
 , separating each Na-site into two symmetrically distinct but similar sites marked as a and b. PS4 tetrahedra are marked in purple, SnS4 tetrahedra in blue and Na-sites in yellow. The ground-state Na-ordering is shown in Supplementary Fig. 2.
, separating each Na-site into two symmetrically distinct but similar sites marked as a and b. PS4 tetrahedra are marked in purple, SnS4 tetrahedra in blue and Na-sites in yellow. The ground-state Na-ordering is shown in Supplementary Fig. 2.

References
-
- Ellis B. L. & Nazar L. F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid State Mater. Sci. 16, 168–177 (2012).
-
- Kim S.-W., Seo D.-H., Ma X., Ceder G. & Kang K. Electrode materials for rechargeable sodium-ion batteries: potential alternatives to current lithium-ion batteries. Adv. Energy Mater. 2, 710–721 (2012).
-
- Pan H., Hu Y.-S. & Chen L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 6, 2338 (2013).
-
- Ponrouch A., Marchante E., Courty M., Tarascon J.-M. & Palacn M. R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 5, 8572 (2012).
-
- Komaba S. et al.. Electrochemical Na insertion and solid electrolyte interphase for hard-carbon electrodes and application to Na-ion batteries. Adv. Funct. Mater. 21, 3859–3867 (2011).
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

