Red Blood Cell Susceptibility to Pneumolysin: CORRELATION WITH MEMBRANE BIOCHEMICAL AND PHYSICAL PROPERTIES
- PMID: 26984406
- PMCID: PMC4858971
- DOI: 10.1074/jbc.M115.691899
Red Blood Cell Susceptibility to Pneumolysin: CORRELATION WITH MEMBRANE BIOCHEMICAL AND PHYSICAL PROPERTIES
Abstract
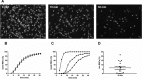
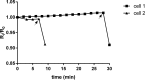
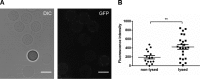
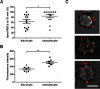
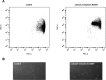
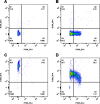
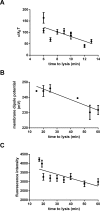
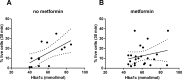
This study investigated the effect of the biochemical and biophysical properties of the plasma membrane as well as membrane morphology on the susceptibility of human red blood cells to the cholesterol-dependent cytolysin pneumolysin, a key virulence factor of Streptococcus pneumoniae, using single cell studies. We show a correlation between the physical properties of the membrane (bending rigidity and surface and dipole electrostatic potentials) and the susceptibility of red blood cells to pneumolysin-induced hemolysis. We demonstrate that biochemical modifications of the membrane induced by oxidative stress, lipid scrambling, and artificial cell aging modulate the cell response to the toxin. We provide evidence that the diversity of response to pneumolysin in diabetic red blood cells correlates with levels of glycated hemoglobin and that the mechanical properties of the red blood cell plasma membrane are altered in diabetes. Finally, we show that diabetic red blood cells are more resistant to pneumolysin and the related toxin perfringolysin O relative to healthy red blood cells. Taken together, these studies indicate that the diversity of cell response to pneumolysin within a population of human red blood cells is influenced by the biophysical and biochemical status of the plasma membrane and the chemical and/or oxidative stress pre-history of the cell.
Keywords: aging; bacterial toxin; bending rigidity; diabetes; dipole potential; erythrocyte; membrane biophysics; oxidative stress; pneumolysin; surface potential.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

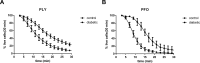


References
-
- Deuticke B. (2003) in Red Cell Membrane Transport in Health and Disease (Bernhardt I., and Ellory J. C., eds) pp. 27–60, Springer, Berlin
-
- Hess J. R., Sparrow R. L., van der Meer P. F., Acker J. P., Cardigan R. A., and Devine D. V. (2009) Red blood cell hemolysis during blood bank storage: using national quality management data to answer basic scientific questions. Transfusion 49, 2599–2603 - PubMed
-
- Waugh R. E., Narla M., Jackson C. W., Mueller T. J., Suzuki T., and Dale G. L. (1992) Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood 79, 1351–1358 - PubMed
-
- Tomaiuolo G., Rossi D., Caserta S., Cesarelli M., and Guido S. (2012) Comparison of two flow-based imaging methods to measure individual red blood cell area and volume. Cytometry A 81, 1040–1047 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

