Amidase Activity of AmiC Controls Cell Separation and Stem Peptide Release and Is Enhanced by NlpD in Neisseria gonorrhoeae
- PMID: 26984407
- PMCID: PMC4865936
- DOI: 10.1074/jbc.M116.715573
Amidase Activity of AmiC Controls Cell Separation and Stem Peptide Release and Is Enhanced by NlpD in Neisseria gonorrhoeae
Abstract
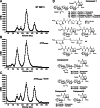
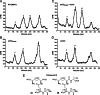
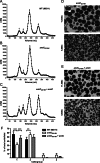
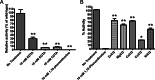
The human-restricted pathogen Neisseria gonorrhoeae encodes a single N-acetylmuramyl-l-alanine amidase involved in cell separation (AmiC), as compared with three largely redundant cell separation amidases found in Escherichia coli (AmiA, AmiB, and AmiC). Deletion of amiC from N. gonorrhoeae results in severely impaired cell separation and altered peptidoglycan (PG) fragment release, but little else is known about how AmiC functions in gonococci. Here, we demonstrated that gonococcal AmiC can act on macromolecular PG to liberate cross-linked and non-cross-linked peptides indicative of amidase activity, and we provided the first evidence that a cell separation amidase can utilize a small synthetic PG fragment as substrate (GlcNAc-MurNAc(pentapeptide)-GlcNAc-MurNAc(pentapeptide)). An investigation of two residues in the active site of AmiC revealed that Glu-229 is critical for both normal cell separation and the release of PG fragments by gonococci during growth. In contrast, Gln-316 has an autoinhibitory role, and its mutation to lysine resulted in an AmiC with increased enzymatic activity on macromolecular PG and on the synthetic PG derivative. Curiously, the same Q316K mutation that increased AmiC activity also resulted in cell separation and PG fragment release defects, indicating that activation state is not the only factor determining normal AmiC activity. In addition to displaying high basal activity on PG, gonococcal AmiC can utilize metal ions other than the zinc cofactor typically used by cell separation amidases, potentially protecting its ability to function in zinc-limiting environments. Thus gonococcal AmiC has distinct differences from related enzymes, and these studies revealed parameters for how AmiC functions in cell separation and PG fragment release.
Keywords: Gram-negative bacteria; NOD-like receptor (NLR); Neisseria gonorrhoeae; amidase; cell separation; cell wall; infectious disease; pathogen-associated molecular pattern (PAMP); peptidoglycan.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Vollmer W. (2008) Structural variation in the glycan strands of bacterial peptidoglycan. FEMS Microbiol. Rev. 32, 287–306 - PubMed
-
- Centers for Disease Control and Prevention (2013) Antibiotic Resistance Threats in the United States, 2013, http://www.cdc.gov/drugresistance/threat-report-2013/
-
- Gregg C. R., Melly M. A., Hellerqvist C. G., Coniglio J. G., and McGee Z. A. (1981) Toxic activity of purified lipopolysaccharide of Neisseria gonorrhoeae for human fallopian tube mucosa. J. Infect. Dis. 143, 432–439 - PubMed
-
- Massari P., Henneke P., Ho Y., Latz E., Golenbock D. T., and Wetzler L. M. (2002) Cutting edge: immune stimulation by neisserial porins is toll-like receptor 2 and MyD88 dependent. J. Immunol. 168, 1533–1537 - PubMed
-
- Melly M. A., McGee Z. A., and Rosenthal R. S. (1984) Ability of monomeric peptidoglycan fragments from Neisseria gonorrhoeae to damage human fallopian tube mucosa. J. Infect. Dis. 149, 378–386 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

