The β-Arrestins: Multifunctional Regulators of G Protein-coupled Receptors
- PMID: 26984408
- PMCID: PMC4861465
- DOI: 10.1074/jbc.R115.713313
The β-Arrestins: Multifunctional Regulators of G Protein-coupled Receptors
Abstract
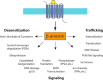
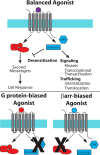
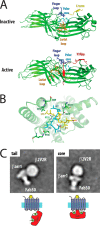
The β-arrestins (βarrs) are versatile, multifunctional adapter proteins that are best known for their ability to desensitize G protein-coupled receptors (GPCRs), but also regulate a diverse array of cellular functions. To signal in such a complex fashion, βarrs adopt multiple conformations and are regulated at multiple levels to differentially activate downstream pathways. Recent structural studies have demonstrated that βarrs have a conserved structure and activation mechanism, with plasticity of their structural fold, allowing them to adopt a wide array of conformations. Novel roles for βarrs continue to be identified, demonstrating the importance of these dynamic regulators of cellular signaling.
Keywords: 7-helix receptor; G protein-coupled receptor (GPCR); arrestin; receptor desensitization; receptor endocytosis; signaling.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., and Lefkowitz R. J. (1990) β-Arrestin: a protein that regulates β-adrenergic receptor function. Science 248, 1547–1550 - PubMed
-
- Wilden U., Wüst E., Weyand I., and Kühn H. (1986) Rapid affinity purification of retinal arrestin (48 kDa protein) via its light-dependent binding to phosphorylated rhodopsin. FEBS Lett. 207, 292–295 - PubMed
-
- Attramadal H., Arriza J. L., Aoki C., Dawson T. M., Codina J., Kwatra M. M., Snyder S. H., Caron M. G., and Lefkowitz R. J. (1992) β-Arrestin2, a novel member of the arrestin/β-arrestin gene family. J. Biol. Chem. 267, 17882–17890 - PubMed
-
- Craft C. M., Whitmore D. H., and Wiechmann A. F. (1994) Cone arrestin identified by targeting expression of a functional family. J. Biol. Chem. 269, 4613–4619 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

