Porcine Reproductive and Respiratory Syndrome Virus Utilizes Nanotubes for Intercellular Spread
- PMID: 26984724
- PMCID: PMC4859731
- DOI: 10.1128/JVI.00036-16
Porcine Reproductive and Respiratory Syndrome Virus Utilizes Nanotubes for Intercellular Spread
Abstract
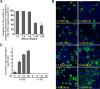
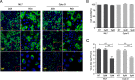
Intercellular nanotube connections have been identified as an alternative pathway for cellular spreading of certain viruses. In cells infected with porcine reproductive and respiratory syndrome virus (PRRSV), nanotubes were observed connecting two distant cells with contiguous membranes, with the core infectious viral machinery (viral RNA, certain replicases, and certain structural proteins) present in/on the intercellular nanotubes. Live-cell movies tracked the intercellular transport of a recombinant PRRSV that expressed green fluorescent protein (GFP)-tagged nsp2. In MARC-145 cells expressing PRRSV receptors, GFP-nsp2 moved from one cell to another through nanotubes in the presence of virus-neutralizing antibodies. Intercellular transport of viral proteins did not require the PRRSV receptor as it was observed in receptor-negative HEK-293T cells after transfection with an infectious clone of GFP-PRRSV. In addition, GFP-nsp2 was detected in HEK-293T cells cocultured with recombinant PRRSV-infected MARC-145 cells. The intercellular nanotubes contained filamentous actin (F-actin) with myosin-associated motor proteins. The F-actin and myosin IIA were identified as coprecipitates with PRRSV nsp1β, nsp2, nsp2TF, nsp4, nsp7-nsp8, GP5, and N proteins. Drugs inhibiting actin polymerization or myosin IIA activation prevented nanotube formation and viral clusters in virus-infected cells. These data lead us to propose that PRRSV utilizes the host cell cytoskeletal machinery inside nanotubes for efficient cell-to-cell spread. This form of virus transport represents an alternative pathway for virus spread, which is resistant to the host humoral immune response.
Importance: Extracellular virus particles transmit infection between organisms, but within infected hosts intercellular infection can be spread by additional mechanisms. In this study, we describe an alternative pathway for intercellular transmission of PRRSV in which the virus uses nanotube connections to transport infectious viral RNA, certain replicases, and certain structural proteins to neighboring cells. This process involves interaction of viral proteins with cytoskeletal proteins that form the nanotube connections. Intercellular viral spread through nanotubes allows the virus to escape the neutralizing antibody response and may contribute to the pathogenesis of viral infections. The development of strategies that interfere with this process could be critical in preventing the spread of viral infection.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures







References
-
- Xu WF, Santini PA, Sullivan JS, He B, Shan MM, Ball SC, Dyer WB, Ketas TJ, Chadburn A, Cohen-Gould L, Knowles DM, Chiu A, Sanders RW, Chen K, Cerutti A. 2009. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat Immunol 10:1008–U1106. doi: 10.1038/ni.1753. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

