Integrated Omics and Computational Glycobiology Reveal Structural Basis for Influenza A Virus Glycan Microheterogeneity and Host Interactions
- PMID: 26984886
- PMCID: PMC5083086
- DOI: 10.1074/mcp.M116.058016
Integrated Omics and Computational Glycobiology Reveal Structural Basis for Influenza A Virus Glycan Microheterogeneity and Host Interactions
Abstract
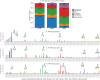
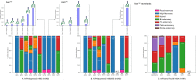
Despite sustained biomedical research effort, influenza A virus remains an imminent threat to the world population and a major healthcare burden. The challenge in developing vaccines against influenza is the ability of the virus to mutate rapidly in response to selective immune pressure. Hemagglutinin is the predominant surface glycoprotein and the primary determinant of antigenicity, virulence and zoonotic potential. Mutations leading to changes in the number of HA glycosylation sites are often reported. Such genetic sequencing studies predict at best the disruption or creation of sequons for N-linked glycosylation; they do not reflect actual phenotypic changes in HA structure. Therefore, combined analysis of glycan micro and macro-heterogeneity and bioassays will better define the relationships among glycosylation, viral bioactivity and evolution. We present a study that integrates proteomics, glycomics and glycoproteomics of HA before and after adaptation to innate immune system pressure. We combined this information with glycan array and immune lectin binding data to correlate the phenotypic changes with biological activity. Underprocessed glycoforms predominated at the glycosylation sites found to be involved in viral evolution in response to selection pressures and interactions with innate immune-lectins. To understand the structural basis for site-specific glycan microheterogeneity at these sites, we performed structural modeling and molecular dynamics simulations. We observed that the presence of immature, high-mannose type glycans at a particular site correlated with reduced accessibility to glycan remodeling enzymes. Further, the high mannose glycans at sites implicated in immune lectin recognition were predicted to be capable of forming trimeric interactions with the immune-lectin surfactant protein-D.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

