Effects of exercise training on urinary tract function after spinal cord injury
- PMID: 26984956
- PMCID: PMC4935767
- DOI: 10.1152/ajprenal.00557.2015
Effects of exercise training on urinary tract function after spinal cord injury
Abstract
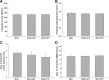
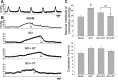
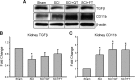
Spinal cord injury (SCI) causes dramatic changes in the quality of life, including coping with bladder dysfunction which requires repeated daily and nightly catheterizations. Our laboratory has recently demonstrated in a rat SCI model that repetitive sensory information generated through task-specific stepping and/or loading can improve nonlocomotor functions, including bladder function (Ward PJ, Herrity AN, Smith RR, Willhite A, Harrison BJ, Petruska JC, Harkema SJ, Hubscher CH. J Neurotrauma 31: 819-833, 2014). To target potential underlying mechanisms, the current study included a forelimb-only exercise group to ascertain whether improvements may be attributed to general activity effects that impact target organ-neural interactions or to plasticity of the lumbosacral circuitry that receives convergent somatovisceral inputs. Male Wistar rats received a T9 contusion injury and were randomly assigned to three groups 2 wk postinjury: quadrupedal locomotion, forelimb exercise, or a nontrained group. Throughout the study (including preinjury), all animals were placed in metabolic cages once a week for 24 h to monitor water intake and urine output. Following the 10-wk period of daily 1-h treadmill training, awake cystometry data were collected and bladder and kidney tissue harvested for analysis. Metabolic cage frequency-volume measurements of voiding and cystometry reveal an impact of exercise training on multiple SCI-induced impairments related to various aspects of urinary tract function. Improvements in both the quadrupedal and forelimb-trained groups implicate underlying mechanisms beyond repetitive sensory information from the hindlimbs driving spinal network excitability of the lumbosacral urogenital neural circuitry. Furthermore, the impact of exercise training on the upper urinary tract (kidney) underscores the health benefit of activity-based training on the entire urinary system within the SCI population.
Keywords: bladder; contusion; kidney; locomotor training.
Copyright © 2016 the American Physiological Society.
Figures




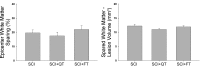
Similar articles
-
Activity-Based Training Alters Penile Reflex Responses in a Rat Model of Spinal Cord Injury.J Sex Med. 2019 Aug;16(8):1143-1154. doi: 10.1016/j.jsxm.2019.05.018. Epub 2019 Jul 2. J Sex Med. 2019. PMID: 31277969
-
Activity-Based Training Reverses Spinal Cord Injury-Induced Changes in Kidney Receptor Densities and Membrane Proteins.J Neurotrauma. 2020 Feb 1;37(3):555-563. doi: 10.1089/neu.2019.6670. Epub 2019 Oct 11. J Neurotrauma. 2020. PMID: 31456470
-
Novel multi-system functional gains via task specific training in spinal cord injured male rats.J Neurotrauma. 2014 May 1;31(9):819-33. doi: 10.1089/neu.2013.3082. Epub 2014 Mar 25. J Neurotrauma. 2014. PMID: 24294909 Free PMC article.
-
Effect of injury severity on lower urinary tract function after experimental spinal cord injury.Prog Brain Res. 2006;152:117-34. doi: 10.1016/S0079-6123(05)52008-9. Prog Brain Res. 2006. PMID: 16198697 Review.
-
The "beneficial" effects of locomotor training after various types of spinal lesions in cats and rats.Prog Brain Res. 2015;218:173-98. doi: 10.1016/bs.pbr.2014.12.009. Epub 2015 Mar 29. Prog Brain Res. 2015. PMID: 25890137 Review.
Cited by
-
Effect of high-intensity exercise training on functional recovery after spinal cord injury.Front Neurol. 2025 Feb 17;16:1442004. doi: 10.3389/fneur.2025.1442004. eCollection 2025. Front Neurol. 2025. PMID: 40035032 Free PMC article. Review.
-
Effect of Different Forms of Activity-Based Recovery Training on Bladder, Bowel, and Sexual Function After Spinal Cord Injury.Arch Phys Med Rehabil. 2021 May;102(5):865-873. doi: 10.1016/j.apmr.2020.11.002. Epub 2020 Dec 3. Arch Phys Med Rehabil. 2021. PMID: 33278365 Free PMC article.
-
Respiratory plasticity following spinal cord injury: perspectives from mouse to man.Neural Regen Res. 2022 Oct;17(10):2141-2148. doi: 10.4103/1673-5374.335839. Neural Regen Res. 2022. PMID: 35259820 Free PMC article. Review.
-
System failure: Systemic inflammation following spinal cord injury.Eur J Immunol. 2024 Jan;54(1):e2250274. doi: 10.1002/eji.202250274. Epub 2023 Oct 19. Eur J Immunol. 2024. PMID: 37822141 Free PMC article. Review.
-
Bladder and Bowel Management in Dogs With Spinal Cord Injury.Front Vet Sci. 2020 Nov 11;7:583342. doi: 10.3389/fvets.2020.583342. eCollection 2020. Front Vet Sci. 2020. PMID: 33263015 Free PMC article. Review.
References
-
- Abbate M, Zoja C, Morigi M, Rottoli D, Angioletti S, Tomasoni S, Zanchi C, Longaretti L, Donadelli R, Remuzzi G. Transforming growth factor-beta1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: a central pathway in progressive glomerulosclerosis. Am J Pathol 161: 2179–2193, 2002. - PMC - PubMed
-
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21: 1371–1383, 2004. - PubMed
-
- Anderson KD, Borisoff JF, Johnson RD, Stiens SA, Elliott SL. The impact of spinal cord injury on sexual function: concerns of the general population. Spinal Cord 45: 328–337, 2007. - PubMed
-
- Basoni C, Nobles M, Grimshaw A, Desgranges C, Davies D, Perretti M, Kramer IM, Genot E. Inhibitory control of TGF-beta1 on the activation of Rap1, CD11b, and transendothelial migration of leukocytes. FASEB J 19: 822–824, 2005. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

