Using the Cancer Risk Management Model to evaluate the health and economic impacts of cytology compared with human papillomavirus DNA testing for primary cervical cancer screening in Canada
- PMID: 26985148
- PMCID: PMC4780581
- DOI: 10.3747/co.23.2991
Using the Cancer Risk Management Model to evaluate the health and economic impacts of cytology compared with human papillomavirus DNA testing for primary cervical cancer screening in Canada
Abstract
Background: In Canada, discussion about changing from cytology to human papillomavirus (hpv) dna testing for primary screening in cervical cancer is ongoing. However, the Canadian Task Force on Preventive Health Care has not yet made a recommendation, concluding that the evidence is insufficient.
Methods: We used the cervical cancer and hpv transmission models of the Cancer Risk Management Model to study the health and economic outcomes of primary cytology compared with hpv dna testing in 14 screening scenarios with varying screening modalities and intervals. Projected cervical cancer cases, deaths, colposcopies, screens, costs, and incremental cost-effectiveness were evaluated. We performed sensitivity analyses for hpv dna test costs.
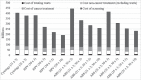
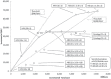
Results: Compared with triennial cytology from age 25, 5-yearly hpv dna screening alone from age 30 resulted in equivalent incident cases and deaths, but 55% (82,000) fewer colposcopies and 43% (1,195,000) fewer screens. At hpv dna screening intervals of 3 years, whether alone or in an age-based sequence with cytology, screening costs are greater, but at intervals of more than 5 years, they are lower. Scenarios on the cost-effectiveness frontier were hpv dna testing alone every 10, 7.5, 5, or 3 years, and triennial cytology starting at age 21 or 25 when combined with hpv dna testing every 3 years.
Conclusions: Changing from cytology to hpv dna testing as the primary screening test for cervical cancer would be an acceptable strategy in Canada with respect to incidence, mortality, screening and diagnostic test volumes.
Keywords: Canada; Human papillomavirus; cervical cancer; cytology; hpvdna; modelling; screening.
Figures
Similar articles
-
Decision-analytic modeling to evaluate the long-term effectiveness and cost-effectiveness of HPV-DNA testing in primary cervical cancer screening in Germany.GMS Health Technol Assess. 2010 Apr 27;6:Doc05. doi: 10.3205/hta000083. GMS Health Technol Assess. 2010. PMID: 21289878 Free PMC article.
-
Screening for Cervical Cancer: A Decision Analysis for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 May. Report No.: 11-05157-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2011 May. Report No.: 11-05157-EF-1. PMID: 22553886 Free Books & Documents. Review.
-
Cost-effectiveness of primary HPV screening for cervical cancer in Germany--a decision analysis.Eur J Cancer. 2011 Jul;47(11):1633-46. doi: 10.1016/j.ejca.2011.03.006. Epub 2011 Apr 7. Eur J Cancer. 2011. PMID: 21482103
-
Cost-effectiveness analysis for Pap smear screening and human papillomavirus DNA testing and vaccination.J Eval Clin Pract. 2011 Dec;17(6):1050-8. doi: 10.1111/j.1365-2753.2010.01453.x. Epub 2011 Jun 16. J Eval Clin Pract. 2011. PMID: 21679279
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
Cited by
-
The OncoSim model: development and use for better decision-making in Canadian cancer control.Curr Oncol. 2017 Dec;24(6):401-406. doi: 10.3747/co.24.3850. Epub 2017 Dec 20. Curr Oncol. 2017. PMID: 29270052 Free PMC article.
-
The Relationship Between Cervicovaginal Infection, Human Papillomavirus Infection and Cervical Intraepithelial Neoplasia in Romanian Women.Diseases. 2025 Jan 16;13(1):18. doi: 10.3390/diseases13010018. Diseases. 2025. PMID: 39851482 Free PMC article.
-
The OncoSim-Breast Cancer Microsimulation Model.Curr Oncol. 2022 Mar 3;29(3):1619-1633. doi: 10.3390/curroncol29030136. Curr Oncol. 2022. PMID: 35323336 Free PMC article.
-
Should We Continue to Perform Pap Smears on Women Who No Longer Have a Cervix?Am J Public Health. 2016 Nov;106(11):1900-1901. doi: 10.2105/AJPH.2016.303411. Am J Public Health. 2016. PMID: 27715296 Free PMC article. No abstract available.
-
Priorities among effective clinical preventive services in British Columbia, Canada.BMC Health Serv Res. 2022 Apr 26;22(1):564. doi: 10.1186/s12913-022-07871-0. BMC Health Serv Res. 2022. PMID: 35473549 Free PMC article.
References
-
- Canadian Partnership Against Cancer (cpac) Cervical Cancer Screening in Canada: Monitoring Program Performance 2009–2011. Toronto, ON: CPAC; 2013.
LinkOut - more resources
Full Text Sources
Other Literature Sources

