Characterization of the Treg Response in the Hepatitis B Virus Hydrodynamic Injection Mouse Model
- PMID: 26986976
- PMCID: PMC4795771
- DOI: 10.1371/journal.pone.0151717
Characterization of the Treg Response in the Hepatitis B Virus Hydrodynamic Injection Mouse Model
Abstract
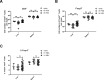
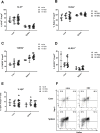
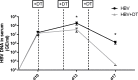
Regulatory T cells (Tregs) play an important role in counter-regulating effector T cell responses in many infectious diseases. However, they can also contribute to the development of T cell dysfunction and pathogen persistence in chronic infections. Tregs have been reported to suppress virus-specific T cell responses in hepatitis B virus (HBV) infection of human patients as well as in HBV animal models. However, the phenotype and expansion of Tregs has so far only been investigated in other infections, but not in HBV. We therefore performed hydrodynamic injections of HBV plasmids into mice and analyzed the Treg response in the spleen and liver. Absolute Treg numbers significantly increased in the liver but not the spleen after HBV injection. The cells were natural Tregs that surprisingly did not show any activation or proliferation in response to the infection. However, they were able to suppress effector T cell responses, as selective depletion of Tregs significantly increased HBV-specific CD8+ T cell responses and accelerated viral antigen clearance. The data implies that natural Tregs infiltrate the liver in HBV infection without further activation or expansion but are still able to interfere with T cell mediated viral clearance.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

