Substrate engagement of integrins α5β1 and αvβ3 is necessary, but not sufficient, for high directional persistence in migration on fibronectin
- PMID: 26987342
- PMCID: PMC4796868
- DOI: 10.1038/srep23258
Substrate engagement of integrins α5β1 and αvβ3 is necessary, but not sufficient, for high directional persistence in migration on fibronectin
Abstract
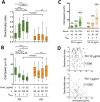
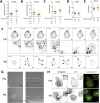
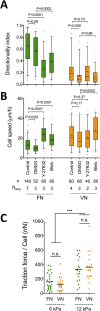
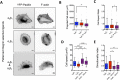
The interplay between specific integrin-mediated matrix adhesion and directional persistence in cell migration is not well understood. Here, we characterized fibroblast adhesion and migration on the extracellular matrix glycoproteins fibronectin and vitronectin, focusing on the role of α5β1 and αvβ3 integrins. Fibroblasts manifested high directional persistence in migration on fibronectin-, but not vitronectin-coated substrates, in a ligand density-dependent manner. Fibronectin stimulated α5β1-dependent organization of the actin cytoskeleton into oriented, ventral stress fibers, and assembly of dynamic, polarized protrusions, characterized as regions free of stress fibers and rich in nascent adhesions at their edge. Such protrusions correlated with persistent, local leading edge advancement, but were not sufficient, nor necessary for directional migration over longer times. Selective blocking of αvβ3 or α5β1 integrins using small molecule integrin antagonists reduced directional persistence on fibronectin, indicating integrin cooperativity in maintaining directionality. On the other hand, patterned substrates, designed to selectively engage either integrin, or their combination, were not sufficient to establish directional migration. Overall, our study demonstrates adhesive coating-dependent regulation of directional persistence in fibroblast migration and challenges the generality of the previously suggested role of β1 and β3 integrins in directional migration.
Figures



References
-
- Ridley A. J. et al.. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003). - PubMed
-
- Kim H.-D. & Peyton S. R. Bio-inspired materials for parsing matrix physicochemical control of cell migration: A Review. Integr. Biol. 4, 37–52 (2012). - PubMed
-
- Geiger B., Spatz J. P. & Bershadsky A. D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 10, 21–33 (2009). - PubMed
-
- Plow E. F., Haas T. A., Zhang L., Loftus J. & Smith J. W. Ligand binding to integrins. J. Biol. Chem. 275, 21788 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

