Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import
- PMID: 26988202
- PMCID: PMC4796820
- DOI: 10.1038/srep23138
Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import
Erratum in
-
Corrigendum: Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import.Sci Rep. 2016 May 9;6:25428. doi: 10.1038/srep25428. Sci Rep. 2016. PMID: 27156930 Free PMC article. No abstract available.
Abstract
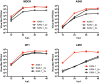
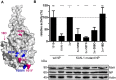
To establish a new lineage in the human population, avian influenza A viruses (AIV) must overcome the intracellular restriction factor MxA. Partial escape from MxA restriction can be achieved when the viral nucleoprotein (NP) acquires the critical human-adaptive amino acid residues 100I/V, 283P, and 313Y. Here, we show that introduction of these three residues into the NP of an avian H5N1 virus renders it genetically unstable, resulting in viruses harboring additional single mutations, including G16D. These substitutions restored genetic stability yet again yielded viruses with varying degrees of attenuation in mammalian and avian cells. Additionally, most of the mutant viruses lost the capacity to escape MxA restriction, with the exception of the G16D virus. We show that MxA escape is linked to attenuation by demonstrating that the three substitutions promoting MxA escape disturbed intracellular trafficking of incoming viral ribonucleoprotein complexes (vRNPs), thereby resulting in impaired nuclear import, and that the additional acquired mutations only partially compensate for this import block. We conclude that for adaptation to the human host, AIV must not only overcome MxA restriction but also an associated block in nuclear vRNP import. This inherent difficulty may partially explain the frequent failure of AIV to become pandemic.
Figures


References
-
- Klenk H. D. Influenza viruses en route from birds to man. Cell Host Microbe 15, 653–654 (2014). - PubMed
-
- Miller M. S. & Palese P. Peering into the crystal ball: influenza pandemics and vaccine efficacy. Cell 157, 294–299 (2014). - PubMed
-
- Hatta M. & Kawaoka Y. The continued pandemic threat posed by avian influenza viruses in Hong Kong. Trends Microbiol. 10, 340–344 (2002). - PubMed
-
- WHO. Influenza at the Human-Animal Interface (HAI) http://www.who.int/influenza/human_animal_interface/en/ (accessed 16 October 2015).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

