Pharmacokinetic modeling of [(18)F]fluorodeoxyglucose (FDG) for premature infants, and newborns through 5-year-olds
- PMID: 26988861
- PMCID: PMC4797375
- DOI: 10.1186/s13550-016-0179-6
Pharmacokinetic modeling of [(18)F]fluorodeoxyglucose (FDG) for premature infants, and newborns through 5-year-olds
Abstract
Background: Absorbed dose estimates for pediatric patients require pharmacokinetics that are, to the extent possible, age-specific. Such age-specific pharmacokinetic data are lacking for many of the diagnostic agents typically used in pediatric imaging. We have developed a pharmacokinetic model of [(18)F]fluorodeoxyglucose (FDG) applicable to premature infants and to 0- (newborns) to 5-year-old patients, which may be used to generate model-derived time-integrated activity coefficients and absorbed dose calculations for these patients.
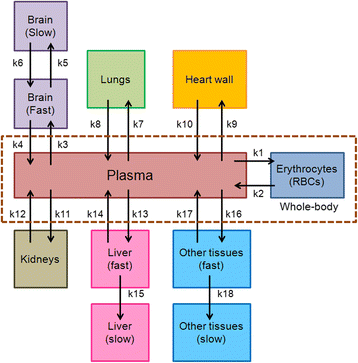
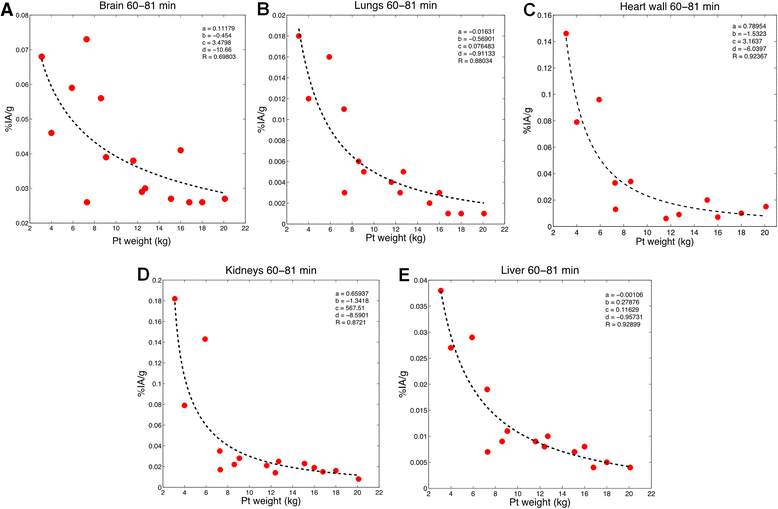
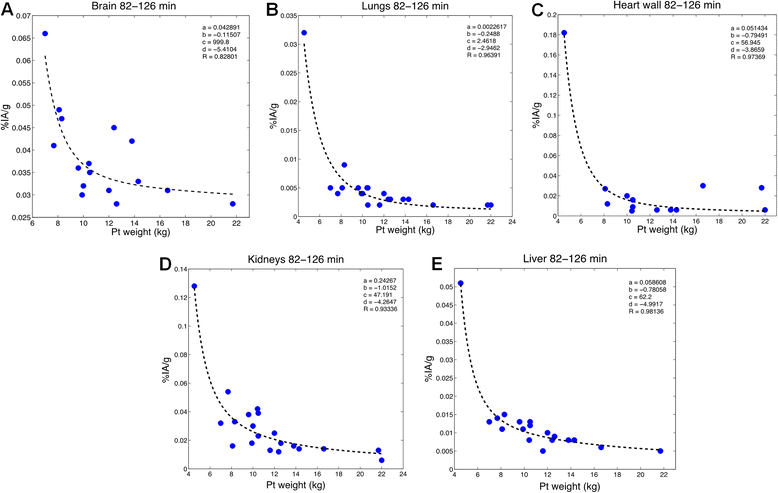
Methods: The FDG compartmental model developed by Hays and Segall for adults was fitted to published data from infants and also to a retrospective data set collected at the Boston Children's Hospital (BCH). The BCH data set was also used to examine the relationship between uptake of FDG in different organs and patient weight or age.
Results: Substantial changes in the structure of the FDG model were required to fit the pediatric data. Fitted rate constants and fractional blood volumes were reduced relative to the adult values.
Conclusions: The pharmacokinetic models developed differ substantially from adult pharmacokinetic (PK) models which can have considerable impact on the dosimetric models for pediatric patients. This approach may be used as a model for estimating dosimetry in children from other radiopharmaceuticals.
Keywords: Compartmental modeling; FDG; Pediatric imaging; Pharmacokinetics.
Figures



References
-
- National Research Council . Health risks from exposure to low levels of ionizing radiation: BEIR VII—phase 2. Washington, DC: National Research Council; 2005. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

