Diversity of Neural Precursors in the Adult Mammalian Brain
- PMID: 26988967
- PMCID: PMC4817799
- DOI: 10.1101/cshperspect.a018838
Diversity of Neural Precursors in the Adult Mammalian Brain
Abstract
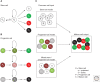
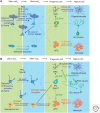
Aided by advances in technology, recent studies of neural precursor identity and regulation have revealed various cell types as contributors to ongoing cell genesis in the adult mammalian brain. Here, we use stem-cell biology as a framework to highlight the diversity of adult neural precursor populations and emphasize their hierarchy, organization, and plasticity under physiological and pathological conditions.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Ahn S, Joyner AL. 2005. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 437: 894–897. - PubMed
-
- Altman J, Bayer SA. 1990. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol 301: 365–381. - PubMed
-
- Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. 2008. The heterogeneity of adult neural stem cells and the emerging complexity of their niche. Cold Spring Harb Symp Quant Biol 73: 357–365. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. 2002. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8: 963–970. - PubMed
-
- Bardehle S, Kruger M, Buggenthin F, Schwausch J, Ninkovic J, Clevers H, Snippert HJ, Theis FJ, Meyer-Luehmann M, Bechmann I, et al. 2013. Live imaging of astrocyte responses to acute injury reveals selective juxtavascular proliferation. Nat Neurosci 16: 580–586. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
