Elucidation of the Roles of Tumor Integrin β1 in the Extravasation Stage of the Metastasis Cascade
- PMID: 26988988
- PMCID: PMC4873393
- DOI: 10.1158/0008-5472.CAN-15-1325
Elucidation of the Roles of Tumor Integrin β1 in the Extravasation Stage of the Metastasis Cascade
Abstract
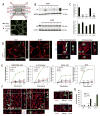
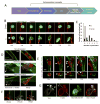
Tumor integrin β1 (ITGB1) contributes to primary tumor growth and metastasis, but its specific roles in extravasation have not yet been clearly elucidated. In this study, we engineered a three-dimensional microfluidic model of the human microvasculature to recapitulate the environment wherein extravasation takes place and assess the consequences of β1 depletion in cancer cells. Combined with confocal imaging, these tools allowed us to decipher the detailed morphology of transmigrating tumor cells and associated endothelial cells in vitro at high spatio-temporal resolution not easily achieved in conventional transmigration assays. Dynamic imaging revealed that β1-depleted cells lacked the ability to sustain protrusions into the subendothelial matrix in contrast with control cells. Specifically, adhesion via α3β1 and α6β1 to subendothelial laminin was a critical prerequisite for successful transmigration. β1 was required to invade past the endothelial basement membrane, whereas its attenuation in a syngeneic tumor model resulted in reduced metastatic colonization of the lung, an effect not observed upon depletion of other integrin alpha and beta subunits. Collectively, our findings in this novel model of the extravasation microenvironment revealed a critical requirement for β1 in several steps of extravasation, providing new insights into the mechanisms underlying metastasis. Cancer Res; 76(9); 2513-24. ©2016 AACR.
©2016 American Association for Cancer Research.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures




References
-
- Heyder C, et al. Realtime visualization of tumor cell/endothelial cell interactions during transmigration across the endothelial barrier. J Cancer Res Clin Oncol. 2002;128:533–8. - PubMed
-
- Haier J. An Intravital Model to Monitor Steps of Metastatic Tumor Cell Adhesion Within the Hepatic Microcirculation. J Gastrointest Surg. 2003;7:507–515. - PubMed
-
- Hynes RO. Integrins: Bidirectional , Allosteric Signaling Machines In their roles as major adhesion receptors , integrins. 2002;110:673–687. - PubMed
-
- Orini MM, et al. THE α3 β1 INTEGRIN IS ASSOCIATED WITH MAMMARY CARCINOMA CELL METASTASIS , INVASION , AND GELATINASE B ( MMP-9 ) ACTIVITY. 2000;342:336–342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

