Trop2 marks transient gastric fetal epithelium and adult regenerating cells after epithelial damage
- PMID: 26989172
- PMCID: PMC4986166
- DOI: 10.1242/dev.131490
Trop2 marks transient gastric fetal epithelium and adult regenerating cells after epithelial damage
Abstract
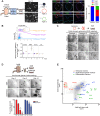
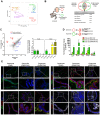
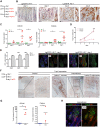
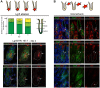
Mouse fetal intestinal progenitors lining the epithelium prior to villogenesis grow as spheroids when cultured ex vivo and express the transmembrane glycoprotein Trop2 as a marker. Here, we report the characterization of Trop2-expressing cells from fetal pre-glandular stomach, growing as immortal undifferentiated spheroids, and their relationship with gastric development and regeneration. Trop2(+) cells generating gastric spheroids differed from adult glandular Lgr5(+) stem cells, but appeared highly related to fetal intestinal spheroids. Although they shared a common spheroid signature, intestinal and gastric fetal spheroid-generating cells expressed organ-specific transcription factors and were committed to intestinal and glandular gastric differentiation, respectively. Trop2 expression was transient during glandular stomach development, being lost at the onset of gland formation, whereas it persisted in the squamous forestomach. Undetectable under homeostasis, Trop2 was strongly re-expressed in glands after acute Lgr5(+) stem cell ablation or following indomethacin-induced injury. These highly proliferative reactive adult Trop2(+) cells exhibited a transcriptome displaying similarity with that of gastric embryonic Trop2(+) cells, suggesting that epithelium regeneration in adult stomach glands involves the partial re-expression of a fetal genetic program.
Keywords: Embryonic; Indomethacin; Lgr5; Spheroids; Stomach; Tacstd2.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Barbera M., di Pietro M., Walker E., Brierley C., MacRae S., Simons B. D., Jones P. H., Stingl J. and Fitzgerald R. C. (2015). The human squamous oesophagus has widespread capacity for clonal expansion from cells at diverse stages of differentiation. Gut 64, 11-19. 10.1136/gutjnl-2013-306171 - DOI - PMC - PubMed
-
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H. J., van Es J. H., Sato T., Stange D. E., Begthel H., van den Born M. et al. (2010). Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25-36. 10.1016/j.stem.2009.11.013 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

