Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound
- PMID: 26989578
- PMCID: PMC4779293
- DOI: 10.1089/wound.2014.0561
Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound
Abstract
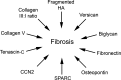
Significance: Fibroblasts play a critical role in normal wound healing. Various extracellular matrix (ECM) components, including collagens, fibrin, fibronectin, proteoglycans, glycosaminoglycans, and matricellular proteins, can be considered potent protagonists of fibroblast survival, migration, and metabolism. Recent Advances: Advances in tissue culture, tissue engineering, and ex vivo models have made the examination and precise measurements of ECM components in wound healing possible. Likewise, the development of specific transgenic animal models has created the opportunity to characterize the role of various ECM molecules in healing wounds. In addition, the recent characterization of new ECM molecules, including matricellular proteins, dermatopontin, and FACIT collagens (Fibril-Associated Collagens with Interrupted Triple helices), further demonstrates our cursory knowledge of the ECM in coordinated wound healing. Critical Issues: The manipulation and augmentation of ECM components in the healing wound is emerging in patient care, as demonstrated by the use of acellular dermal matrices, tissue scaffolds, and wound dressings or topical products bearing ECM proteins such as collagen, hyaluronan (HA), or elastin. Once thought of as neutral structural proteins, these molecules are now known to directly influence many aspects of cellular wound healing. Future Directions: The role that ECM molecules, such as CCN2, osteopontin, and secreted protein, acidic and rich in cysteine, play in signaling homing of fibroblast progenitor cells to sites of injury invites future research as we continue investigating the heterotopic origin of certain populations of fibroblasts in a healing wound. Likewise, research into differently sized fragments of the same polymeric ECM molecule is warranted as we learn that fragments of molecules such as HA and tenascin-C can have opposing effects on dermal fibroblasts.
Figures





References
-
- Stoker M, O'Neill C, Berryman S, Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer 1968;3:683–693 - PubMed
-
- Alberts J, Johnson B, Lewis A. The Extracellular Matrix of Animals. New York: Garland Science, 2002
-
- Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science 1971;173:548–550 - PubMed
-
- Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–162 - PubMed
-
- Sorrell JM, Baber MA, Caplan AI. Clonal characterization of fibroblasts in the superficial layer of the adult human dermis. Cell Tissue Res 2007;327:499–510 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

