Evaluation of Chitosan-Tripolyphosphate Nanoparticles as a p-shRNA Delivery Vector: Formulation, Optimization and Cellular Uptake Study
- PMID: 26989641
- PMCID: PMC4792291
- DOI: 10.1166/jnd.2013.1027
Evaluation of Chitosan-Tripolyphosphate Nanoparticles as a p-shRNA Delivery Vector: Formulation, Optimization and Cellular Uptake Study
Abstract
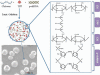
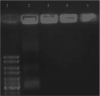
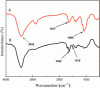
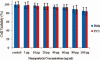
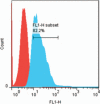
Polysaccharides (especially chitosan) have recently attracted much attention as gene therapy delivery vehicles for their unique properties such as biocompatibility, biodegradability, low toxicity, and controlled release. Nanoparticles have strong potential as a carrier of plasmid short hairpin RNA (p-shRNA). This study aimed to find the optimum conditions for obtaining Chitosan/triphosphate (TPP)/p-shRNA nanoparticles by the ionic gelation method, and investigating the cellular uptake of the optimized nanoparticles. After applying the central composite design of response surface methodology (RSM), the optimum conditions for preparation of nanoparticles with small size and high loading efficiency were: chitosan/TPP ratio = 10, pH = 5.5 and N/P ratio = 11. The resulting nanoparticles had an average size of 172.8 ± 7 nm and loading efficiency of 71.5 ± 5%. SEM images showed spherical and smooth nanoparticles. The nanoparticles complexed with p-shRNA and may protect it against nuclease digestion. Cytotoxicity studies with HeLa and PC3 human cancer cells demonstrated that chitosan/TPP nanoparticles had low toxicity. Cellular uptake studies using HeLa cells showed that the nanoparticles entered the cells (cellular uptake) and delivered DNA, probably due to their favorable Zeta potential (approximately +28 mV) and small size.
Keywords: Cellular Uptake; Chitosan; Gene Delivery; Nanoparticle; Response Surface Methodology (RSM); p-shRNA.
Figures



References
-
- Barichello JM, Morishita M, Takayama K, Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Development and Industrial Pharmacy. 1999;25:471. - PubMed
-
- Duan Y, Zheng J, Han S, Wu Y, Wang Y, Li D, Kong D, Yu Y. A tumor targeted gene vector modified with G250 monoclonal antibody for gene therapy. J. Controlled Rel. 2008;127:173. - PubMed
-
- El-Aneed A. An overview of current delivery systems in cancer gene therapy. J. Controlled Rel. 2004;94:1. - PubMed
-
- Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Advanced Drug Delivery Reviews. 2006;58:467. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
