Antiobesity and Antidiabetes Effects of a Cudrania tricuspidata Hydrophilic Extract Presenting PTP1B Inhibitory Potential
- PMID: 26989693
- PMCID: PMC4775776
- DOI: 10.1155/2016/8432759
Antiobesity and Antidiabetes Effects of a Cudrania tricuspidata Hydrophilic Extract Presenting PTP1B Inhibitory Potential
Abstract
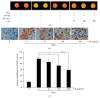
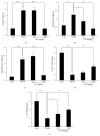
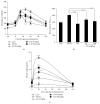
Diabetes and obesity represent the major health problems and the most age-related metabolic diseases. Protein-tyrosine phosphatase 1B (PTP1B) has emerged as an important regulator of insulin signal transduction and is regarded as a pharmaceutical target for metabolic disorders. To find novel natural materials presenting therapeutic activities against diabetes and obesity, we screened various herb extracts using a chip screening allowing the determination of PTP1B inhibitory effects of the tested compounds using insulin receptor (IR) as the substrate. Cudrania tricuspidata leaves (CTe) had a strong inhibitory effect on PTP1B activity and substantially inhibited fat accumulation in 3T3-L1 cells. CTe was orally administrated to diet-induced obesity (DIO) mice once daily for 3 weeks after which changes in glucose, insulin metabolism, and fat accumulation were examined. Hepatic enzyme markers (aspartate aminotransferase, AST, and alanine aminotransferase, ALT) and total fat mass and triglyceride levels decreased in CTe-treated mice, whereas body weight and total cholesterol concentration slightly decreased. CTe increased the phosphorylation of IRS-1 and Akt in liver tissue. Furthermore, CTe treatment significantly lowered blood glucose levels and improved insulin secretion in DIO mice. Our results strongly suggest that CTe may represent a promising therapeutic substance against diabetes and obesity.
Figures


References
-
- Requejo O. H., Rodriguez M. C. R. Nutrition and cancer. Nutrición Hospitalaria. 2015;32(supplement 1):67–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

