Quiescent and Active Tear Protein Profiles to Predict Vernal Keratoconjunctivitis Reactivation
- PMID: 26989694
- PMCID: PMC4773530
- DOI: 10.1155/2016/9672082
Quiescent and Active Tear Protein Profiles to Predict Vernal Keratoconjunctivitis Reactivation
Abstract
Objective: Vernal keratoconjunctivitis (VKC) is a chronic recurrent bilateral inflammation of the conjunctiva associated with atopy. Several inflammatory and tissue remodeling factors contribute to VKC disease. The aim is to provide a chip-based protein analysis in tears from patients suffering from quiescent or active VKC.
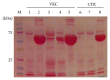
Methods: This study cohort included 16 consecutive patients with VKC and 10 controls. Participants were subjected to clinical assessment of ocular surface and tear sampling. Total protein quantification, total protein sketch, and protein array (sixty protein candidates) were evaluated.
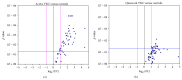
Results: An overall increased Fluorescent Intensity expression was observed in VKC arrays. Particularly, IL1β, IL15, IL21, Eotaxin2, TACE, MIP1α, MIP3α, NCAM1, ICAM2, βNGF, NT4, BDNF, βFGF, SCF, MMP1, and MMP2 were increased in quiescent VKC. Of those candidates, only IL1β, IL15, IL21, βNGF, SCF, MMP2, Eotaxin2, TACE, MIP1α, MIP3α, NCAM1, and ICAM2 were increased in both active and quiescent VKC. Finally, NT4, βFGF, and MMP1 were highly increased in active VKC.
Conclusion: A distinct "protein tear-print" characterizes VKC activity, confirming some previously reported factors and highlighting some new candidates common to quiescent and active states. Those candidates expressed in quiescent VKC might be considered as predictive indicators of VKC reactivation and/or exacerbation out-of-season.
Figures



References
-
- Uchio E., Ono S. Y., Ikezawa Z., et al. Tear levels of IFN-gamma, interleukin (IL) 2, IL4 and IL5 in patients with vernal keratoconjunctivitis, atopic keratitis and allergic conjunctivitis. Clinical & Experimental Allergy. 2000;30(1):103–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

