Reorganization of chromosome architecture in replicative cellular senescence
- PMID: 26989773
- PMCID: PMC4788486
- DOI: 10.1126/sciadv.1500882
Reorganization of chromosome architecture in replicative cellular senescence
Abstract
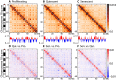
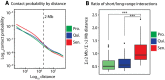
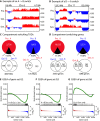
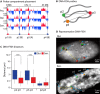
Replicative cellular senescence is a fundamental biological process characterized by an irreversible arrest of proliferation. Senescent cells accumulate a variety of epigenetic changes, but the three-dimensional (3D) organization of their chromatin is not known. We applied a combination of whole-genome chromosome conformation capture (Hi-C), fluorescence in situ hybridization, and in silico modeling methods to characterize the 3D architecture of interphase chromosomes in proliferating, quiescent, and senescent cells. Although the overall organization of the chromatin into active (A) and repressive (B) compartments and topologically associated domains (TADs) is conserved between the three conditions, a subset of TADs switches between compartments. On a global level, the Hi-C interaction matrices of senescent cells are characterized by a relative loss of long-range and gain of short-range interactions within chromosomes. Direct measurements of distances between genetic loci, chromosome volumes, and chromatin accessibility suggest that the Hi-C interaction changes are caused by a significant reduction of the volumes occupied by individual chromosome arms. In contrast, centromeres oppose this overall compaction trend and increase in volume. The structural model arising from our study provides a unique high-resolution view of the complex chromosomal architecture in senescent cells.
Keywords: DNA FISH; Hi-C; cellular senescence; centromere; chromatin; chromosome architecture; chromosome conformation capture; genome organization; long-range interactions; telomere.
Figures

References
-
- Muñoz-Espín D., Cañamero M., Maraver A., Gómez-López G., Contreras J., Murillo-Cuesta S., Rodríguez-Baeza A., Varela-Nieto I., Ruberte J., Collado M., Serrano M., Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013). - PubMed
-
- Storer M., Mas A., Robert-Moreno A., Pecoraro M., Ortells M. C., Di Giacomo V., Yosef R., Pilpel N., Krizhanovsky V., Sharpe J., Keyes W. M., Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

