Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics
- PMID: 26989783
- PMCID: PMC4788475
- DOI: 10.1126/sciadv.1501340
Reconsidering plant memory: Intersections between stress recovery, RNA turnover, and epigenetics
Abstract
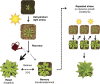
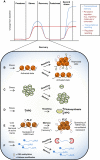
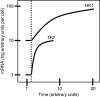
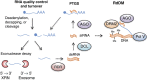
Plants grow in dynamic environments where they can be exposed to a multitude of stressful factors, all of which affect their development, yield, and, ultimately, reproductive success. Plants are adept at rapidly acclimating to stressful conditions and are able to further fortify their defenses by retaining memories of stress to enable stronger or more rapid responses should an environmental perturbation recur. Indeed, one mechanism that is often evoked regarding environmental memories is epigenetics. Yet, there are relatively few examples of such memories; neither is there a clear understanding of their duration, considering the plethora of stresses in nature. We propose that this field would benefit from investigations into the processes and mechanisms enabling recovery from stress. An understanding of stress recovery could provide fresh insights into when, how, and why environmental memories are created and regulated. Stress memories may be maladaptive, hindering recovery and affecting development and potential yield. In some circumstances, it may be advantageous for plants to learn to forget. Accordingly, the recovery process entails a balancing act between resetting and memory formation. During recovery, RNA metabolism, posttranscriptional gene silencing, and RNA-directed DNA methylation have the potential to play key roles in resetting the epigenome and transcriptome and in altering memory. Exploration of this emerging area of research is becoming ever more tractable with advances in genomics, phenomics, and high-throughput sequencing methodology that will enable unprecedented profiling of high-resolution stress recovery time series experiments and sampling of large natural populations.
Keywords: DNA methylation; RNA metabolism; RNA turnover; abiotic stress; arabidopsis; epigenetics; gene silencing; plant memory; plants; stress recovery.
Figures


References
-
- Bruce T. J. A., Matthes M. C., Napier J. A., Pickett J. A., Stressful “memories” of plants: Evidence and possible mechanisms. Plant Sci. 173, 603–608 (2007).
-
- Conrath U., Molecular aspects of defence priming. Trends Plant Sci. 16, 524–531 (2011). - PubMed
-
- Walter J., Nagy L., Hein R., Rascher U., Beierkuhnlein C., Willner E., Jentsch A., Do plants remember drought? Hints towards a drought-memory in grasses. Environ. Exp. Bot. 71, 34–40 (2011).
-
- Baldwin I. T., Schmelz E. A., Immunological “memory” in the induced accumulation of nicotine in wild tobacco. Ecology 77, 236–246 (1996).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

