Metabolomic analysis of survival in carbohydrate pre-fed pigs subjected to shock and polytrauma
- PMID: 26989839
- PMCID: PMC5577932
- DOI: 10.1039/c5mb00637f
Metabolomic analysis of survival in carbohydrate pre-fed pigs subjected to shock and polytrauma
Abstract
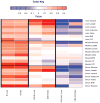
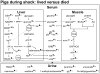
Hemorrhagic shock, a result of extensive blood loss, is a dominant factor in battlefield morbidity and mortality. Early rodent studies in hemorrhagic shock reported carbohydrate feeding prior to the induction of hemorrhagic shock decreased mortality. When repeated in our laboratory with a porcine model, carbohydrate pre-feed resulted in a 60% increase in death rate following hemorrhagic shock with trauma when compared to fasted animals (15/32 or 47% vs. 9/32 or 28%). In an attempt to explain the unexpected death rate for pre-fed animals, we further investigated the metabolic profiles of pre-fed non-survivors (n = 15) across 4 compartments (liver, muscle, serum, and urine) at specific time intervals (pre-shock, shock, and resuscitation) and compared them to pre-fed survivors (n = 17). As hypothesized, pre-fed pigs that died as a result of hemorrhage and trauma showed differences in their metabolic and physiologic profiles at all time intervals and in all compartments when compared to pre-fed survivors. Our data suggest that, although all animals were subjected to the same shock and trauma protocol, non-survivors exhibited altered carbohydrate processing as early as the pre-shock sampling point. This was evident in (for example) the higher levels of ATP and markers of greater anabolic activity in the muscle at the pre-shock time point. Based on the metabolic findings, we propose two mechanisms that connect pre-fed status to a higher death rate: (1) animals that die are more susceptible to opening of the mitochondrial permeability transition pore, a major factor in ischemia/reperfusion injury; and (2) loss of fasting-associated survival mechanisms in pre-fed animals.
Figures






Similar articles
-
A four-compartment metabolomics analysis of the liver, muscle, serum, and urine response to polytrauma with hemorrhagic shock following carbohydrate prefeed.PLoS One. 2015 Apr 14;10(4):e0124467. doi: 10.1371/journal.pone.0124467. eCollection 2015. PLoS One. 2015. PMID: 25875111 Free PMC article.
-
Metabolic networks in a porcine model of trauma and hemorrhagic shock demonstrate different control mechanism with carbohydrate pre-feed.BMC Emerg Med. 2015 Jul 1;15:13. doi: 10.1186/s12873-015-0038-1. BMC Emerg Med. 2015. PMID: 26130247 Free PMC article.
-
Preinjury Fed State Alters the Physiologic Response in a Porcine Model of Hemorrhagic Shock and Polytrauma.Shock. 2015 Aug;44 Suppl 1:103-13. doi: 10.1097/SHK.0000000000000324. Shock. 2015. PMID: 25565641
-
Metabolomics classifies phase of care and identifies risk for mortality in a porcine model of multiple injuries and hemorrhagic shock.J Trauma Acute Care Surg. 2012 Aug;73(2 Suppl 1):S147-55. doi: 10.1097/TA.0b013e3182609821. J Trauma Acute Care Surg. 2012. PMID: 22847086
-
Mouse Injury Model of Polytrauma and Shock.Methods Mol Biol. 2018;1717:1-15. doi: 10.1007/978-1-4939-7526-6_1. Methods Mol Biol. 2018. PMID: 29468579 Free PMC article. Review.
Cited by
-
Metabolomics and Precision Medicine in Trauma: The State of the Field.Shock. 2018 Jul;50(1):5-13. doi: 10.1097/SHK.0000000000001093. Shock. 2018. PMID: 29280924 Free PMC article.
-
Establishment and evaluation of rat trauma hemorrhagic liver injury model.Int J Clin Exp Pathol. 2017 Jul 1;10(7):7340-7349. eCollection 2017. Int J Clin Exp Pathol. 2017. PMID: 31966574 Free PMC article.
References
-
- Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG. Inflammation, Host Response to Injury Large-Scale Collaborative Research Program. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. - DOI - PMC - PubMed
-
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Crit Care Med. 1985;13:818–829. - PubMed
-
- Baker SP, o’Neill B, Haddon W, Jr, Long WB. Journal of Trauma and Acute Care Surgery. 1974;14:187–196. - PubMed
-
- DUNHAM CM, SIEGEL JH, WEIRETER L, FABIAN M, GOODARZI S, GUADALUPI P, GETTINGS L, LINBERG SE, VARY TC. Crit Care Med. 1991;19:231–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

