Effects of short-chain acyl-CoA dehydrogenase on cardiomyocyte apoptosis
- PMID: 26989860
- PMCID: PMC4929297
- DOI: 10.1111/jcmm.12828
Effects of short-chain acyl-CoA dehydrogenase on cardiomyocyte apoptosis
Abstract
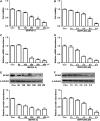
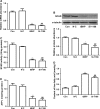
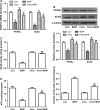
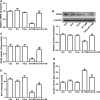
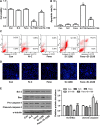
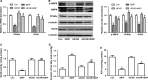
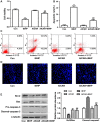
Short-chain acyl-CoA dehydrogenase (SCAD), a key enzyme of fatty acid β-oxidation, plays an important role in cardiac hypertrophy. However, its effect on the cardiomyocyte apoptosis remains unknown. We aimed to determine the role of SCAD in tert-butyl hydroperoxide (tBHP)-induced cardiomyocyte apoptosis. The mRNA and protein expression of SCAD were significantly down-regulated in the cardiomyocyte apoptosis model. Inhibition of SCAD with siRNA-1186 significantly decreased SCAD expression, enzyme activity and ATP content, but obviously increased the content of free fatty acids. Meanwhile, SCAD siRNA treatment triggered the same apoptosis as cardiomyocytes treated with tBHP, such as the increase in cell apoptotic rate, the activation of caspase3 and the decrease in the Bcl-2/Bax ratio, which showed that SCAD may play an important role in primary cardiomyocyte apoptosis. The changes of phosphonate AMP-activated protein kinase α (p-AMPKα) and Peroxisome proliferator-activated receptor α (PPARα) in cardiomyocyte apoptosis were consistent with that of SCAD. Furthermore, PPARα activator fenofibrate and AMPKα activator AICAR treatment significantly increased the expression of SCAD and inhibited cardiomyocyte apoptosis. In conclusion, for the first time our findings directly demonstrated that SCAD may be as a new target to prevent cardiomyocyte apoptosis through the AMPK/PPARα/SCAD signal pathways.
Keywords: AMP-activated protein kinase; cardiomyocyte apoptosis; energy metabolism•; peroxisome proliferator-activated receptor α; short-chain acyl-CoA dehydrogenase; tert-butyl hydroperoxide.
© 2016 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures


Similar articles
-
Effects of ERK1/2/PPARα/SCAD signal pathways on cardiomyocyte hypertrophy induced by insulin-like growth factor 1 and phenylephrine.Life Sci. 2015 Mar 1;124:41-9. doi: 10.1016/j.lfs.2015.01.015. Epub 2015 Jan 28. Life Sci. 2015. PMID: 25636810
-
Changes in short-chain acyl-coA dehydrogenase during rat cardiac development and stress.J Cell Mol Med. 2015 Jul;19(7):1672-88. doi: 10.1111/jcmm.12541. Epub 2015 Mar 8. J Cell Mol Med. 2015. PMID: 25753319 Free PMC article.
-
[Effects of short-chain acyl-CoA dehydrogenase on human umbilical vein endothelial cell apoptosis].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019 Jun;31(6):756-761. doi: 10.3760/cma.j.issn.2095-4352.2019.06.019. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019. PMID: 31315737 Chinese.
-
AMPK activation enhances PPARα activity to inhibit cardiac hypertrophy via ERK1/2 MAPK signaling pathway.Arch Biochem Biophys. 2011 Jul;511(1-2):1-7. doi: 10.1016/j.abb.2011.04.010. Epub 2011 Apr 21. Arch Biochem Biophys. 2011. PMID: 21530483
-
Molecular pathogenesis of a novel mutation, G108D, in short-chain acyl-CoA dehydrogenase identified in subjects with short-chain acyl-CoA dehydrogenase deficiency.Hum Genet. 2010 Jun;127(6):619-28. doi: 10.1007/s00439-010-0822-7. Epub 2010 Apr 8. Hum Genet. 2010. PMID: 20376488 Review.
Cited by
-
SGLT2 inhibitors: role in protective reprogramming of cardiac nutrient transport and metabolism.Nat Rev Cardiol. 2023 Jul;20(7):443-462. doi: 10.1038/s41569-022-00824-4. Epub 2023 Jan 6. Nat Rev Cardiol. 2023. PMID: 36609604 Review.
-
Fenofibrate Ameliorates Hepatic Ischemia/Reperfusion Injury in Mice: Involvements of Apoptosis, Autophagy, and PPAR-α Activation.PPAR Res. 2021 Feb 1;2021:6658944. doi: 10.1155/2021/6658944. eCollection 2021. PPAR Res. 2021. PMID: 33603777 Free PMC article.
References
-
- Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010; 72: 19–44. - PubMed
-
- Zhang Y, Herman B. Apoptosis and successful aging. Mech Ageing Dev. 2002; 123: 563–5. - PubMed
-
- Lee Y, Gustafsson AB. Role of apoptosis in cardiovascular disease. Apoptosis. 2009; 14: 536–48. - PubMed
-
- Nishida K, Otsu K. Cell death in heart failure. Circ J. 2008; 72 (Suppl. A): A17–21. - PubMed
-
- Lu FH, Fu SB, Leng X, et al Role of the calcium‐sensing receptor in cardiomyocyte apoptosis via the sarcoplasmic reticulum and mitochondrial death pathway in cardiac hypertrophy and heart failure. Cell Physiol Biochem. 2013; 31: 728–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

