Dynamic Mechanical and Nanofibrous Topological Combinatory Cues Designed for Periodontal Ligament Engineering
- PMID: 26989897
- PMCID: PMC4798756
- DOI: 10.1371/journal.pone.0149967
Dynamic Mechanical and Nanofibrous Topological Combinatory Cues Designed for Periodontal Ligament Engineering
Erratum in
-
Correction: Dynamic mechanical and nanofibrous topological combinatory cues designed for periodontal ligament engineering.PLoS One. 2020 Jan 24;15(1):e0228475. doi: 10.1371/journal.pone.0228475. eCollection 2020. PLoS One. 2020. PMID: 31978171 Free PMC article.
Abstract
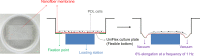
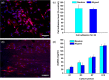
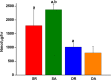
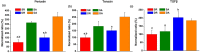
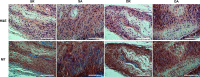
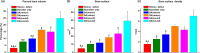
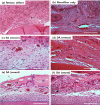
Complete reconstruction of damaged periodontal pockets, particularly regeneration of periodontal ligament (PDL) has been a significant challenge in dentistry. Tissue engineering approach utilizing PDL stem cells and scaffolding matrices offers great opportunity to this, and applying physical and mechanical cues mimicking native tissue conditions are of special importance. Here we approach to regenerate periodontal tissues by engineering PDL cells supported on a nanofibrous scaffold under a mechanical-stressed condition. PDL stem cells isolated from rats were seeded on an electrospun polycaprolactone/gelatin directionally-oriented nanofiber membrane and dynamic mechanical stress was applied to the cell/nanofiber construct, providing nanotopological and mechanical combined cues. Cells recognized the nanofiber orientation, aligning in parallel, and the mechanical stress increased the cell alignment. Importantly, the cells cultured on the oriented nanofiber combined with the mechanical stress produced significantly stimulated PDL specific markers, including periostin and tenascin with simultaneous down-regulation of osteogenesis, demonstrating the roles of topological and mechanical cues in altering phenotypic change in PDL cells. Tissue compatibility of the tissue-engineered constructs was confirmed in rat subcutaneous sites. Furthermore, in vivo regeneration of PDL and alveolar bone tissues was examined under the rat premaxillary periodontal defect models. The cell/nanofiber constructs engineered under mechanical stress showed sound integration into tissue defects and the regenerated bone volume and area were significantly improved. This study provides an effective tissue engineering approach for periodontal regeneration-culturing PDL stem cells with combinatory cues of oriented nanotopology and dynamic mechanical stretch.
Conflict of interest statement
Figures




Similar articles
-
Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium.Acta Biomater. 2015 Oct;25:240-52. doi: 10.1016/j.actbio.2015.07.023. Epub 2015 Jul 15. Acta Biomater. 2015. PMID: 26188325
-
Participation of periodontal ligament cells with regeneration of alveolar bone.J Periodontol. 2001 Mar;72(3):314-23. doi: 10.1902/jop.2001.72.3.314. J Periodontol. 2001. PMID: 11327058
-
Collagen functionalized bioactive nanofiber matrices for osteogenic differentiation of mesenchymal stem cells: bone tissue engineering.J Biomed Nanotechnol. 2014 Feb;10(2):287-98. doi: 10.1166/jbn.2014.1753. J Biomed Nanotechnol. 2014. PMID: 24738337
-
The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration.J Periodontal Res. 2017 Dec;52(6):965-974. doi: 10.1111/jre.12477. Epub 2017 Jun 21. J Periodontal Res. 2017. PMID: 28635007 Review.
-
Periodontal tissue engineering: defining the triad.Int J Oral Maxillofac Implants. 2013 Nov-Dec;28(6):e461-71. doi: 10.11607/jomi.te26. Int J Oral Maxillofac Implants. 2013. PMID: 24278956 Review.
Cited by
-
Combined Delivery of Two Different Bioactive Factors Incorporated in Hydroxyapatite Microcarrier for Bone Regeneration.Tissue Eng Regen Med. 2020 Oct;17(5):607-624. doi: 10.1007/s13770-020-00257-5. Epub 2020 Aug 16. Tissue Eng Regen Med. 2020. PMID: 32803541 Free PMC article.
-
Bone remodeling induced by mechanical forces is regulated by miRNAs.Biosci Rep. 2018 Jul 2;38(4):BSR20180448. doi: 10.1042/BSR20180448. Print 2018 Aug 31. Biosci Rep. 2018. PMID: 29844019 Free PMC article. Review.
-
Correction: Dynamic mechanical and nanofibrous topological combinatory cues designed for periodontal ligament engineering.PLoS One. 2020 Jan 24;15(1):e0228475. doi: 10.1371/journal.pone.0228475. eCollection 2020. PLoS One. 2020. PMID: 31978171 Free PMC article.
-
Evaluation of the periodontal regenerative properties of patterned human periodontal ligament stem cell sheets.J Periodontal Implant Sci. 2017 Dec;47(6):402-415. doi: 10.5051/jpis.2017.47.6.402. Epub 2017 Dec 31. J Periodontal Implant Sci. 2017. PMID: 29333326 Free PMC article.
-
Potential of Trilayered Gelatin/Polycaprolactone Nanofibers for Periodontal Regeneration: An In Vitro Study.Int J Mol Sci. 2025 Jan 15;26(2):672. doi: 10.3390/ijms26020672. Int J Mol Sci. 2025. PMID: 39859386 Free PMC article.
References
-
- Bartold PM, Xiao Y, Lyngstaadas SP, Paine ML, Snead ML. (2006) Principles and applications of cell delivery systems for periodontal regeneration. Periodontol 2000. 41: 123–135. - PubMed
-
- Cho MI, Matsuda N, Lin WL, Moshier A, Ramakrishnan PR. (1992) In vitro formation of mineralized nodules by periodontal ligament cells from the rat. Calcif Tissue Int. 50: 459–467. - PubMed
-
- Ziegler N, Alonso A, Steinberg T, Woodnutt D, Kohl A, Müssig E, et al. (2010) Mechano-transduction in periodontal ligament cells identifies activated states of MAP-kinases p42/44 and p38-stress kinase as a mechanism for MMP-13 expression. BMC Cell Biol. 11: 10 10.1186/1471-2121-11-10 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

