Vav1 Regulates Mesenchymal Stem Cell Differentiation Decision Between Adipocyte and Chondrocyte via Sirt1
- PMID: 26990002
- PMCID: PMC7780243
- DOI: 10.1002/stem.2365
Vav1 Regulates Mesenchymal Stem Cell Differentiation Decision Between Adipocyte and Chondrocyte via Sirt1
Abstract
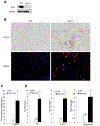
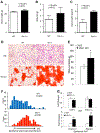
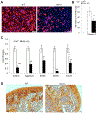
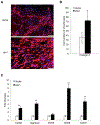
Mesenchymal stem cells (MSCs) are multipotent stromal cells residing in the bone marrow. MSCs have the potential to differentiate to adipocytes, chondrocytes, and other types of cells. In this study, we investigated the molecular mechanism that controls MSC cell fate decisions for differentiation. We found that Vav1, a guanine nucleotide exchange factor for Rho GTPase, was highly expressed in MSCs. Interestingly, loss of Vav1 in MSCs led to spontaneous adipogenic but impaired chondrogenic differentiation, and accordingly Vav1 null mice displayed an increase in fat content and a decrease in cartilage. Conversely, ectopic expression of Vav1 in MSCs reversed this phenotype, and led to enhanced MSC differentiation into chondrocyte but retarded adipogenesis. Mechanistically, loss of Vav1 reduced the level of Sirt1, which was responsible for an increase of acetylated PPARγ. As acetylation activates PPARγ, it increased C/EBPα expression and promoted adipogenesis. On the other hand, loss of Vav1 resulted in an increase of acetylated Sox9, a target of Sirt1. As acetylation represses Sox9 activity, it led to a dramatic reduction of collagen 2α1, a key regulator in chondrocyte differentiation. Finally, we found that Vav1 regulates Sirt1 in MSCs through Creb. Together this study reveals a novel function of Vav1 in regulating MSC cell fate decisions for differentiation through Sirt1. Sirt1 deacetylates PPARγ and Sox9, two key mediators that control adipocyte and chondrocyte differentiation. The acetylation status of PPARγ and Sox9 has opposite effects on its activity, thereby controlling cell fate decision. Stem Cells 2016;34:1934-1946.
Keywords: Adipogenesis; Chondrogenesis; MSC; Sirt1; Vav1.
2016 AlphaMed Press.
Conflict of interest statement
DISCLOSURE
The authors indicate no potential conflicts of interest.
Figures


References
-
- Backesjo CM, Li Y, Lindgren U et al. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res 2006; 21:993–1002. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

