Pasireotide Therapy of Multiple Endocrine Neoplasia Type 1-Associated Neuroendocrine Tumors in Female Mice Deleted for an Men1 Allele Improves Survival and Reduces Tumor Progression
- PMID: 26990064
- PMCID: PMC4870877
- DOI: 10.1210/en.2015-1965
Pasireotide Therapy of Multiple Endocrine Neoplasia Type 1-Associated Neuroendocrine Tumors in Female Mice Deleted for an Men1 Allele Improves Survival and Reduces Tumor Progression
Abstract
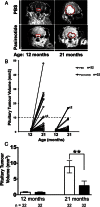
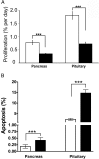
Pasireotide, a somatostatin analog, is reported to have anti-proliferative effects in neuroendocrine tumors (NETs). We therefore assessed the efficacy of pasireotide for treating pancreatic and pituitary NETs that develop in a mouse model of multiple endocrine neoplasia type 1 (MEN1). Men1(+/-) mice were treated from age 12 mo with 40 mg/kg pasireotide long-acting release formulation, or PBS, intramuscularly monthly for 9 mo. The Men1(+/-) mice had magnetic resonance imaging at 12 and 21 mo, and from 20 mo oral 5-bromo-2-deoxyuridine for 1 mo, to assess tumor development and proliferation, respectively. NETs were collected at age 21 mo, and proliferation and apoptosis assessed by immunohistochemistry and TUNEL assays, respectively. Pasireotide-treated Men1(+/-) mice had increased survival (pasireotide, 80.9% vs PBS, 65.2%; P < .05), with fewer mice developing pancreatic NETs (pasireotide, 86.9% vs PBS, 96.9%; P < .05) and smaller increases in pituitary NET volumes (pre-treated vs post-treated, 0.803 ± 0.058 mm(3) vs 2.872 ± 0.728 mm(3) [pasireotide] compared with 0.844 ± 0.066 mm(3) vs 8.847 ±1.948 mm(3) [PBS]; P < .01). In addition, pasireotide-treated mice had fewer pancreatic NETs compared with PBS-treated mice (2.36 ± 0.25 vs 3.72 ± 0.32, respectively; P < .001), with decreased proliferation in pancreatic NETs (pasireotide, 0.35 ± 0.03% vs PBS, 0.78 ± 0.08%; P < .0001) and pituitary NETs (pasireotide, 0.73 ±0.07% vs PBS, 1.81 ± 0.15%; P < .0001), but increased apoptosis in pancreatic NETs (pasireotide, 0.42 ± 0.05% vs PBS, 0.19 ± 0.03%; P < .001) and pituitary NETs (pasireotide, 14.75 ± 1.58% vs PBS, 2.35 ± 0.44%; P < .001). Thus, pasireotide increased survival and inhibited pancreatic and pituitary NET growth, thereby indicating its potential as an anti-proliferative and pro-apoptotic therapy.
Figures




References
-
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. - PubMed
-
- Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: A cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006;91:4769–4775. - PubMed
-
- Daly AF, Vanbellinghen JF, Khoo SK, et al. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: Analysis in 73 families. J Clin Endocrinol Metab. 2007;92:1891–1896. - PubMed
-
- Yates CJ, Newey PJ, Thakker RV. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol. 2015;3(11):895–905. - PubMed
-
- Yates CJ, Lines KE, Thakker RV. Molecular genetic advances in pituitary tumor development. Expert Rev Endocrinol Metab. 2015;10:35–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

