Insights into the mechanisms underlying colonic motor patterns
- PMID: 26990133
- PMCID: PMC4967752
- DOI: 10.1113/JP271919
Insights into the mechanisms underlying colonic motor patterns
Abstract
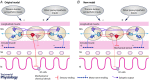
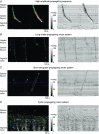
In recent years there have been significant technical and methodological advances in our ability to record the movements of the gastrointestinal tract. This has led to significant changes in our understanding of the different types of motor patterns that exist in the gastrointestinal tract (particularly the large intestine) and in our understanding of the mechanisms underlying their generation. Compared with other tubular smooth muscle organs, a rich variety of motor patterns occurs in the large intestine. This reflects a relatively autonomous nervous system in the gut wall, which has its own unique population of sensory neurons. Although the enteric nervous system can function independently of central neural inputs, under physiological conditions bowel motility is influenced by the CNS: if spinal pathways are disrupted, deficits in motility occur. The combination of high resolution manometry and video imaging has improved our knowledge of the range of motor patterns and provided some insight into the neural and mechanical factors underlying propulsion of contents. The neural circuits responsible for the generation of peristalsis and colonic migrating motor complexes have now been identified to lie within the myenteric plexus and do not require inputs from the mucosa or submucosal ganglia for their generation, but can be modified by their activity. This review will discuss the recent advances in our understanding of the different patterns of propagating motor activity in the large intestine of mammals and how latest technologies have led to major changes in our understanding of the mechanisms underlying their generation.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures







References
-
- Adler HF, Atkinson AJ & Ivy AC (1941). A study of the motility of the human colon: an explanation of dysynergia of the colon, or of the ‘unstable colon’. Am J Dig Dis 8, 197–202.
-
- Anderson KD (2004). Targeting recovery: priorities of the spinal cord‐injured population. J Neurotrauma 21, 1371–1383. - PubMed
-
- Arkwright JW, Underhill ID, Maunder SA, Blenman N, Szczesniak MM, Wiklendt L, Cook IJ, Lubowski DZ & Dinning PG (2009). Design of a high‐sensor count fibre optic manometry catheter for in‐vivo colonic diagnostics. Opt Express 17, 22423–22431. - PubMed
-
- Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ & Cook IJ (2001). Prolonged multi‐point recording of colonic manometry in the unprepared human colon: Providing insight into potentially relevant pressure wave parameters. Am J Gastroenterol 96, 1838–1848. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

