Vertebrate Claudin/PMP22/EMP22/MP20 family protein TMEM47 regulates epithelial cell junction maturation and morphogenesis
- PMID: 26990309
- PMCID: PMC5322768
- DOI: 10.1002/dvdy.24404
Vertebrate Claudin/PMP22/EMP22/MP20 family protein TMEM47 regulates epithelial cell junction maturation and morphogenesis
Abstract
Background: TMEM47 is the vertebrate orthologue of C. elegans VAB-9, a tetraspan adherens junction protein in the PMP22/EMP/Claudin family of proteins. VAB-9 regulates cell morphology and adhesion in C. elegans and TMEM47 is expressed during kidney development and regulates the activity of Fyn. The conserved functions of VAB-9 and TMEM47 are not well understood.
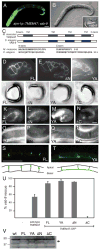
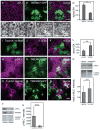
Results: expression of TMEM47 in C. elegans functionally rescues vab-9 mutations. Unlike Claudins, expression of TMEM47 in L fibroblasts does not generate tight junction strands; instead, membrane localization requires E-cadherin expression. Temporally, TMEM47 localizes at cell junctions first with E-cadherin before ZO-1 colocalization and in polarized epithelia, TMEM47 colocalizes with adherens junction proteins. By immunoprecipitation, TMEM47 associates with classical adherens junction proteins, but also with tight junction proteins Par6B and aPKCλ. Over-expression of TMEM47 in MDCK cells decreases apical surface area, increases activated myosin light chain at cell-cell contacts, disrupts cell polarity and morphology, delays cell junction reassembly following calcium switch, and selectively interferes with tight junction assembly. Reduced TMEM47 expression results in opposite phenotypes.
Conclusions: TMEM47 regulates the localization of a subset of tight junction proteins, associated actomyosin structures, cell morphology, and participates in developmental transitions from adherens to tight junctions. Developmental Dynamics 245:653-666, 2016. © 2016 Wiley Periodicals, Inc.
Keywords: Par6B; aPKCλ; actomyosin; adherens junction; cellular junctions; epithelial morphogenesis; tight junction.
© 2016 Wiley Periodicals, Inc.
Figures





References
-
- Bruggeman LA, Martinka S, Simske JS. Expression of TM4SF10, a Claudin/EMP/PMP22 family cell junction protein, during mouse kidney development and podocyte differentiation. Dev Dyn. 2007;236:596–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

