Streptococcus pneumoniae Cell-Wall-Localized Phosphoenolpyruvate Protein Phosphotransferase Can Function as an Adhesin: Identification of Its Host Target Molecules and Evaluation of Its Potential as a Vaccine
- PMID: 26990554
- PMCID: PMC4798226
- DOI: 10.1371/journal.pone.0150320
Streptococcus pneumoniae Cell-Wall-Localized Phosphoenolpyruvate Protein Phosphotransferase Can Function as an Adhesin: Identification of Its Host Target Molecules and Evaluation of Its Potential as a Vaccine
Abstract
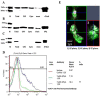
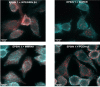
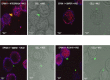
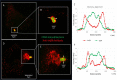
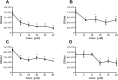
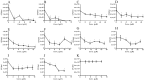
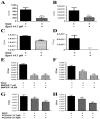
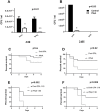
In Streptococcus pneumonia, phosphoenolpyruvate protein phosphotransferase (PtsA) is an intracellular protein of the monosaccharide phosphotransferase systems. Biochemical and immunostaining methods were applied to show that PtsA also localizes to the bacterial cell-wall. Thus, it was suspected that PtsA has functions other than its main cytoplasmic enzymatic role. Indeed, recombinant PtsA and anti-rPtsA antiserum were shown to inhibit adhesion of S. pneumoniae to cultured human lung adenocarcinoma A549 cells. Screening of a combinatorial peptide library expressed in a filamentous phage with rPtsA identified epitopes that were capable of inhibiting S. pneumoniae adhesion to A549 cells. The insert peptides in the phages were sequenced, and homologous sequences were found in human BMPER, multimerin1, protocadherin19, integrinβ4, epsin1 and collagen type VIIα1 proteins, all of which can be found in A549 cells except the latter. Six peptides, synthesized according to the homologous sequences in the human proteins, specifically bound rPtsA in the micromolar range and significantly inhibited pneumococcal adhesion in vitro to lung- and tracheal-derived cell lines. In addition, the tested peptides inhibited lung colonization after intranasal inoculation of mice with S. pneumoniae. Immunization with rPtsA protected the mice against a sublethal intranasal and a lethal intravenous pneumococcal challenge. In addition, mouse anti rPtsA antiserum reduced bacterial virulence in the intravenous inoculation mouse model. These findings showed that the surface-localized PtsA functions as an adhesin, PtsA binding peptides derived from its putative target molecules can be considered for future development of therapeutics, and rPtsA should be regarded as a candidate for vaccine development.
Conflict of interest statement
Figures


References
-
- Hammitt LL, Bulkow LR, Singleton RJ, Nuorti JP, Hummel KB, Miernyk KM, et al. Repeat revaccination with 23-valent pneumococcal polysaccharide vaccine among adults aged 55–74 years living in Alaska: no evidence of hyporesponsiveness. Vaccine. 2011;29(12):2287–95. Epub 2011/01/25. 10.1016/j.vaccine.2011.01.029 . - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

