The differential effects of 1,25-dihydroxyvitamin D3 on Salmonella-induced interleukin-8 and human beta-defensin-2 in intestinal epithelial cells
- PMID: 26990648
- PMCID: PMC4908287
- DOI: 10.1111/cei.12792
The differential effects of 1,25-dihydroxyvitamin D3 on Salmonella-induced interleukin-8 and human beta-defensin-2 in intestinal epithelial cells
Abstract
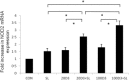
Salmonellosis or Salmonella, one of the most common food-borne diseases, remains a major public health problem worldwide. Intestinal epithelial cells (IECs) play an essential role in the mucosal innate immunity of the host to defend against the invasion of Salmonella by interleukin (IL)-8 and human β-defensin-2 (hBD-2). Accumulated research has unravelled important roles of vitamin D in the regulation of innate immunity. Therefore, we investigated the effects of 1,25-dihydroxyvitamin D3 (1,25D3) on Salmonella-induced innate immunity in IECs. We demonstrate that pretreatment of 1,25D3 results in suppression of Salmonella-induced IL-8 but enhancement of hBD-2, either protein secretion and mRNA expression, in IECs. Furthermore, 1,25D3 enhanced Salmonella-induced membranous recruitment of nucleotide oligomerization domain (NOD2) and its mRNA expression and activation of protein kinase B (Akt), a downstream effector of phosphoinositide 3-kinase (PI3K). Inhibition of the PI3K/Akt signal counteracted the suppressive effect of 1,25D3 on Salmonella-induced IL-8 expression, while knock-down of NOD2 by siRNA diminished the enhanced hBD-2 expression. These data suggest differential regulation of 1,25D3 on Salmonella-induced IL-8 and hBD-2 expression in IECs via PI3K/Akt signal and NOD2 protein expression, respectively. Active vitamin D-enhanced anti-microbial peptide in Salmonella-infected IECs protected the host against infection, while modulation of proinflammatory responses by active vitamin D prevented the host from the detrimental effects of overwhelming inflammation. Thus, active vitamin D-induced innate immunity in IECs enhances the host's protective mechanism, which may provide an alternative therapy for invasive Salmonella infection.
Keywords: Salmonella; human beta-defensin-2; interleukin; intestinal epithelia; vitamin D.
© 2016 British Society for Immunology.
Figures




References
-
- Glynn MK, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo FJ. Emergence of multidrug‐resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med 1998; 338:1333–8. - PubMed
-
- Parry CM. Antimicrobial drug resistance in Salmonella enterica . Curr Opin Infect Dis 2003; 16:467–72. - PubMed
-
- Lauderdale TL, Aarestrup FM, Chen PC et al Multidrug resistance among different serotypes of clinical Salmonella isolates in Taiwan. Diagn Microbiol Infect Dis 2006; 55:149–55. - PubMed
-
- Huang FC, Li Q, Cherayil BJ. A phosphatidyl‐inositol‐3‐kinase‐dependent anti‐inflammatory pathway activated by Salmonella in epithelial cells. FEMS Microbiol Lett 2005; 243:265–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

