Identification of a novel agrin-dependent pathway in cell signaling and adhesion within the erythroid niche
- PMID: 26990660
- PMCID: PMC4947663
- DOI: 10.1038/cdd.2016.10
Identification of a novel agrin-dependent pathway in cell signaling and adhesion within the erythroid niche
Abstract
Establishment of cell-cell adhesion is crucial in embryonic development as well as within the stem cell niches of an adult. Adhesion between macrophages and erythroblasts is required for the formation of erythroblastic islands, specialized niches where erythroblasts proliferate and differentiate to produce red blood cells throughout life. The Eph family is the largest known family of receptor tyrosine kinases (RTKs) and controls cell adhesion, migration, invasion and morphology by modulating integrin and adhesion molecule activity and by modifying the actin cytoskeleton. Here, we identify the proteoglycan agrin as a novel regulator of Eph receptor signaling and characterize a novel mechanism controlling cell-cell adhesion and red cell development within the erythroid niche. We demonstrate that agrin induces clustering and activation of EphB1 receptors on developing erythroblasts, leading to the activation of α5β1 integrins. In agreement, agrin knockout mice display severe anemia owing to defective adhesion to macrophages and impaired maturation of erythroid cells. These results position agrin-EphB1 as a novel key signaling couple regulating cell adhesion and erythropoiesis.
Figures


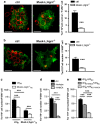
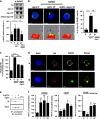
References
-
- Bohme B, VandenBos T, Cerretti DP, Park LS, Holtrich U, Rubsamen-Waigmann H et al. Cell-cell adhesion mediated by binding of membrane-anchored ligand LERK-2 to the EPH-related receptor human embryonal kinase 2 promotes tyrosine kinase activity. J Biol Chem 1996; 271: 24747–24752. - PubMed
-
- Wang HU, Anderson DJ. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron 1997; 18: 383–396. - PubMed
-
- Palmer A, Klein R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev 2003; 17: 1429–1450. - PubMed
-
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 2006; 4: 111–121. - PubMed
-
- Wang Z, Miura N, Bonelli A, Mole P, Carlesso N, Olson DP et al. Receptor tyrosine kinase, EphB4 (HTK), accelerates differentiation of select human hematopoietic cells. Blood 2002; 99: 2740–2747. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

