Aggregation of Trp > Glu point mutants of human gamma-D crystallin provides a model for hereditary or UV-induced cataract
- PMID: 26991007
- PMCID: PMC4941774
- DOI: 10.1002/pro.2924
Aggregation of Trp > Glu point mutants of human gamma-D crystallin provides a model for hereditary or UV-induced cataract
Abstract
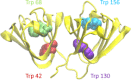
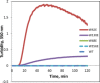
Numerous mutations and covalent modifications of the highly abundant, long-lived crystallins of the eye lens cause their aggregation leading to progressive opacification of the lens, cataract. The nature and biochemical mechanisms of the aggregation process are poorly understood, as neither amyloid nor native-state polymers are commonly found in opaque lenses. The βγ-crystallin fold contains four highly conserved buried tryptophans, which can be oxidized to more hydrophilic products, such as kynurenine, upon UV-B irradiation. We mimicked this class of oxidative damage using Trp→Glu point mutants of human γD-crystallin. Such substitutions may represent a model of UV-induced photodamage-introduction of a charged group into the hydrophobic core generating "denaturation from within." The effects of Trp→Glu substitutions were highly position dependent. While each was destabilizing, only the two located in the bottom of the double Greek key fold-W42E and W130E-yielded robust aggregation of partially unfolded intermediates at 37°C and pH 7. The αB-crystallin chaperone suppressed aggregation of W130E, but not W42E, indicating distinct aggregation pathways from damage in the N-terminal vs C-terminal domain. The W130E aggregates had loosely fibrillar morphology, yet were nonamyloid, noncovalent, showed little surface hydrophobicity, and formed at least 20°C below the melting temperature of the native β-sheets. These features are most consistent with domain-swapped polymerization. Aggregation of partially destabilized crystallins under physiological conditions, as occurs in this class of point mutants, could provide a simple in vitro model system for drug discovery and optimization.
Keywords: amyloid; cataract; crystallin; oxidative damage; protein aggregation; protein misfolding; unfolding intermediate.
© 2016 The Protein Society.
Figures










References
-
- Facts about cataract (2009) National Eye Institute. https://nei.nih.gov/health/cataract/cataract_facts.
-
- Frick KD, Gower EW, Kempen JH, Wolff JL (2007) Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol 125:544–550. - PubMed
-
- Tielsch JM, Kempen JH, Congdon N, Friedman DS (2008) Vision probelms in the U.S.: prevalence of adult visual impairment and age‐related eye disease in America, p. 22. Prevent Blindness America and the National Eye Institute.
-
- Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, Hu LD, Ouyang H, Patel SH, Jin X, Lin DN, Wu F, Flagg K, Cai HM, Li G, Cao GQ, Lin Y, Chen D, Wen C, Chung C, Wang YD, Qiu A, Yeh E, Wang WQ, Hu X, Grob S, Abagyan R, Su ZG, Tjondro HC, Zhao XJ, Luo HR, Hou R, Perry JJP, Gao WW, Kozak I, Granet D, Li YR, Sun XD, Wang J, Zhang LF, Liu YZ, Yan YB, Zhang K (2015) Lanosterol reverses protein aggregation in cataracts. Nature 523:607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

