Anti-Inflammatory Effects of Acupuncture Stimulation via the Vagus Nerve
- PMID: 26991319
- PMCID: PMC4798687
- DOI: 10.1371/journal.pone.0151882
Anti-Inflammatory Effects of Acupuncture Stimulation via the Vagus Nerve
Abstract
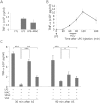
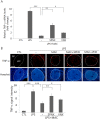
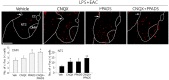
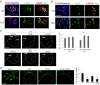
Although acupuncture therapy is widely used in traditional Asian medicine for the treatment of diverse internal organ disorders, its underlying biological mechanisms are largely unknown. Here, we investigated the functional involvement of acupuncture stimulation (AS) in the regulation of inflammatory responses. TNF-α production in mouse serum, which was induced by lipopolysaccharide (LPS) administration, was decreased by manual acupuncture (MAC) at the zusanli acupoint (stomach36, ST36). In the spleen, TNF-α mRNA and protein levels were also downregulated by MAC and were recovered by using a splenic neurectomy and a vagotomy. c-Fos, which was induced in the nucleus tractus solitarius (NTS) and dorsal motor nucleus of the vagus nerve (DMV) by LPS and electroacupuncture (EAC), was further increased by focal administration of the AMPA receptor blocker CNQX and the purinergic receptor antagonist PPADS. TNF-α levels in the spleen were decreased by CNQX and PPADS treatments, implying the involvement of inhibitory neuronal activity in the DVC. In unanesthetized animals, both MAC and EAC generated c-Fos induction in the DVC neurons. However, MAC, but not EAC, was effective in decreasing splenic TNF-α production. These results suggest that the therapeutic effects of acupuncture may be mediated through vagal modulation of inflammatory responses in internal organs.
Conflict of interest statement
Figures


References
-
- Leake R, Broderick JE. Treatment of efficacy of acupuncture: a review of the research literature. Integrative Med 1999;1: 107–115,.
-
- Jänig W. The integrative action of the autonomic nervous system, pp 362–374.: Cambridge University Press; Cambridge, England, 2006.
-
- Kavoussi B, Ross BE. The neuroimmune basis of anti-inflammatory acupuncture. Integr Cancer Ther 2007; 6:251–257. - PubMed
-
- Nishijo K, Mori H, Yosikawa K, Yazawa K. Decreased heart rate by acupuncture stimulation in humans via facilitation of cardiac vagal activity and suppression of cardiac sympathetic nerve. Neurosci Lett 1997; 227:165–168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

