Distinct Hypnotic Recoveries After Infusions of Methoxycarbonyl Etomidate and Cyclopropyl Methoxycarbonyl Metomidate: The Role of the Metabolite
- PMID: 26991617
- PMCID: PMC4800831
- DOI: 10.1213/ANE.0000000000001146
Distinct Hypnotic Recoveries After Infusions of Methoxycarbonyl Etomidate and Cyclopropyl Methoxycarbonyl Metomidate: The Role of the Metabolite
Abstract
Background: Methoxycarbonyl etomidate (MOC-etomidate) and cyclopropyl methoxycarbonyl metomidate (CPMM) are rapidly metabolized "soft" etomidate analogs. CPMM's duration of hypnotic effect is context insensitive, whereas MOC-etomidate's is not. In this study, we tested the hypothesis that CPMM's effect is context insensitive because, unlike MOC-etomidate, its metabolite fails to reach physiologically important concentrations in vivo even with prolonged continuous infusion.
Methods: We compared the potencies with which MOC-etomidate and CPMM activate α1(L264T)β3γ2 γ-aminobutyric acid type A receptors and induce loss-of-righting reflexes (i.e., produce hypnosis) in tadpoles with those of their metabolites (MOC-etomidate's carboxylic acid metabolite [MOC-ECA] and CPMM's carboxylic acid metabolite [CPMM-CA], respectively). We measured metabolite concentrations in the blood and cerebrospinal fluid of Sprague-Dawley rats on CPMM infusion and compared them with those achieved with MOC-etomidate infusion. We measured the rates with which brain tissue from Sprague-Dawley rats metabolize MOC-etomidate and CPMM.
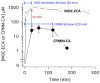
Results: Both analogs and their metabolites enhanced γ-aminobutyric acid type A receptor function and induced loss-of-righting reflexes in a concentration-dependent manner. However, in these 2 assays, CPMM-CA's potency relative to its parent hypnotic was approximately 1:4900 and 1:1900, respectively, whereas MOC-ECA's was only approximately 1:415 and 1:390, respectively. With 2-hour CPMM infusions, CPMM-CA reached respective concentrations in the blood and cerebrospinal fluid that were 2 and >3 orders of magnitude lower than that which produced hypnosis. CPMM was metabolized by the brain tissue at a rate that is approximately 1/15th that of MOC-etomidate.
Conclusions: Hypnotic recovery after CPMM administration is context insensitive because its metabolite does not accumulate to hypnotic levels in the central nervous system. This reflects the very large potency ratio between CPMM and CPMM-CA and the resistance of CPMM to metabolism by esterases present in the brain.
Conflict of interest statement
See Disclosures for Author Conflicts of Interest.
Figures

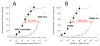
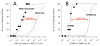

References
-
- Bodor N. Recent advances in retrometabolic drug design and targeting approaches. Pharmazie. 2000;55:163–6. - PubMed
-
- Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, Grosse CM, Hermann D. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: remifentanil (GI87084B) Anesth Analg. 1993;77:1031–40. - PubMed
-
- Rosow C. Remifentanil: A unique opioid analgesic. Anesthesiology. 1993;79:875–6. - PubMed
-
- Sum CY, Yacobi A, Kartzinel R, Stampfli H, Davis CS, Lai CM. Kinetics of esmolol, an ultra-short-acting beta blocker, and of its major metabolite. Clin Pharmacol Ther. 1983;34:427–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

