Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy
- PMID: 26991794
- PMCID: PMC4917796
- DOI: 10.1111/bcp.12938
Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug-induced liver injury case-control study in Italy
Abstract
Aim: Drug-induced liver injury is one of the most serious adverse drug reactions and the most frequent reason for restriction of indications or withdrawal of drugs. Some nonsteroidal anti-inflammatory drugs (NSAIDs) were withdrawn from the market because of serious hepatotoxicity. We estimated the risk of acute and serious liver injury associated with the use of nimesulide and other NSAIDs, with a prevalence of use greater than or equal to 5%.
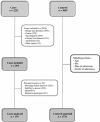
Methods: This is a multicentre case-control study carried out in nine Italian hospitals from October 2010 to January 2014. Cases were adults, with a diagnosis of acute liver injury. Controls presented acute clinical disorders not related to chronic conditions, not involving the liver. Adjusted odds ratio (ORs) with 95% confidence interval (CI) were calculated initially with a bivariate and then multivariate analysis.
Results: We included 179 cases matched to 1770 controls. Adjusted OR for acute serious liver injury associated with all NSAIDs was 1.69, 95% CI 1.21-2.37. Thirty cases were exposed to nimesulide (adjusted OR 2.10, 95% CI 1.28-3.47); the risk increased according to the length of exposure (OR > 30 days: 12.55, 95% CI 1.73-90.88) and to higher doses (OR 10.69, 95% CI 4.02-28.44). Risk of hepatotoxicity was increased also for ibuprofen, used both at recommended dosages (OR 1.92, 95% CI 1.13-3.26) and at higher doses (OR 3.73, 95% CI 1.11-12.46) and for ketoprofen ≥ 150 mg (OR 4.65, 95% CI 1.33-10.00).
Conclusion: Among all NSAIDs, nimesulide is associated with the higher risk, ibuprofen and high doses of ketoprofen are also associated with a modestly increased risk of hepatotoxicity.
Keywords: DILI; NSAIDs; case-control study; liver injury; nimesulide.
© 2016 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures
References
-
- Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137: 947–54. - PubMed
-
- Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med 2007; 262: 393–401. - PubMed
-
- Bechmann LP, Manka P, Best J, Saner FH, Paul A, Canbay A, et al. Drug‐induced liver injury as predominant cause of acute liver failure in a monocenter study. Dtsch Med Wochenschr 2014; 139: 878–82. - PubMed
-
- Watkins PB, Seeff LB. Drug‐induced liver injury: summary of a single topic clinical research conference. Hepatology 2006; 43: 618–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical