Augmented Pain Processing in Primary and Secondary Somatosensory Cortex in Fibromyalgia: A Magnetoencephalography Study Using Intra-Epidermal Electrical Stimulation
- PMID: 26992095
- PMCID: PMC4798786
- DOI: 10.1371/journal.pone.0151776
Augmented Pain Processing in Primary and Secondary Somatosensory Cortex in Fibromyalgia: A Magnetoencephalography Study Using Intra-Epidermal Electrical Stimulation
Abstract
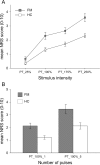
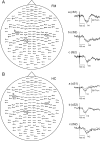
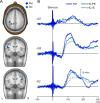
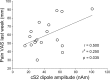
The aim of this study was to investigate augmented pain processing in the cortical somatosensory system in patients with fibromyalgia (FM). Cortical evoked responses were recorded in FM (n = 19) and healthy subjects (n = 21) using magnetoencephalography after noxious intra-epidermal electrical stimulation (IES) of the hand dorsum (pain rating 6 on a numeric rating scale, perceptually-equivalent). In addition, healthy subjects were stimulated using the amplitude corresponding to the average stimulus intensity rated 6 in patients with FM (intensity-equivalent). Quantitative sensory testing was performed on the hand dorsum or thenar muscle (neutral site) and over the trapezius muscle (tender point), using IES (thresholds, ratings, temporal summation of pain, stimulus-response curve) and mechanical stimuli (threshold, ratings). Increased amplitude of cortical responses was found in patients with FM as compared to healthy subjects. These included the contralateral primary (S1) and bilateral secondary somatosensory cortices (S2) in response to intensity-equivalent stimuli and the contralateral S1 and S2 in response to perceptually-equivalent stimuli. The amplitude of the contralateral S2 response in patients with FM was positively correlated with average pain intensity over the last week. Quantitative sensory testing results showed that patients with FM were more sensitive to painful IES as well as to mechanical stimulation, regardless of whether the stimulation site was the hand or the trapezius muscle. Interestingly, the slope of the stimulus-response relationship as well as temporal summation of pain in response to IES was not different between groups. Together, these results suggest that the observed pain augmentation in response to IES in patients with FM could be due to sensitization or disinhibition of the cortical somatosensory system. Since the S2 has been shown to play a role in higher-order functions, further studies are needed to clarify the role of augmented S2 response in clinical characteristics of FM.
Conflict of interest statement
Figures

References
-
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–72. . - PubMed
-
- Berglund B, Harju EL, Kosek E, Lindblom U. Quantitative and qualitative perceptual analysis of cold dysesthesia and hyperalgesia in fibromyalgia. Pain. 2002;96(1–2):177–87. - PubMed
-
- Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70(1):41–51. . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

