Granulocyte colony-stimulating factor (G-CSF) upregulates β1 integrin and increases migration of human trophoblast Swan 71 cells via PI3K and MAPK activation
- PMID: 26992288
- PMCID: PMC5338037
- DOI: 10.1016/j.yexcr.2016.03.005
Granulocyte colony-stimulating factor (G-CSF) upregulates β1 integrin and increases migration of human trophoblast Swan 71 cells via PI3K and MAPK activation
Abstract
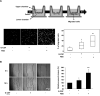
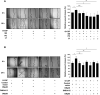
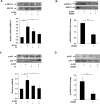
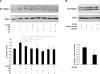
Multiple cytokines and growth factors expressed at the fetal-maternal interface are involved in the regulation of trophoblast functions and placental growth, but the role of G-CSF has not been completely established. Based on our previous study showing that G-CSF increases the activity of matrix metalloproteinase-2 and the release of vascular endothelial growth factor in Swan 71 human trophoblast cells, in this work we explore the possible contribution of G-CSF to cell migration and the G-CSF-triggered signaling pathway. We found that G-CSF induced morphological changes on actin cytoskeleton consistent with a migratory cell phenotype. G-CSF also up-regulated the expression levels of β1 integrin and promoted Swan 71 cell migration. By using selective pharmacological inhibitors and dominant negative mutants we showed that PI3K, Erk 1/2 and p38 pathways are required for promoting Swan 71 cell motility. It was also demonstrated that PI3K behaved as an upstream regulator of Erk 1/2 and p38 MAPK. In addition, the increase of β1 integrin expression was dependent on PI3K activation. In conclusion, our results indicate that G-CSF stimulates β1 integrin expression and Swan 71 cell migration by activating PI3K and MAPK signaling pathways, suggesting that G-CSF should be considered as an additional regulatory factor that contributes to a successful embryo implantation and to the placenta development.
Keywords: G-CSF; MAPK; Migration; PI3K; Swan 71 cells; β1 Integrin.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. - PubMed
-
- van de Geijn GJ, Aarts LH, Erkeland SJ, Prasher JM, Touw IP. Granulocyte colony-stimulating factor and its receptor in normal hematopoietic cell development and myeloid disease. Rev. Physiol. Biochem. Pharmacol. 2003;149:53–71. - PubMed
-
- Touw IP, van de Geijn GJ. Granulocyte colony-stimulating factor and its receptor in normal myeloid cell development, leukemia and related blood cell disorders. Front. Biosci. 2007;12:800–815. - PubMed
-
- Liongue C, Wright C, Russell AP, Ward AC. Granulocyte colony-stimulating factor receptor: stimulating granulopoiesis and much more. Int. J. Biochem. Cell. Biol. 2009;41:2372–2375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

