Rat Prostate Tumor Cells Progress in the Bone Microenvironment to a Highly Aggressive Phenotype
- PMID: 26992916
- PMCID: PMC4796808
- DOI: 10.1016/j.neo.2016.01.007
Rat Prostate Tumor Cells Progress in the Bone Microenvironment to a Highly Aggressive Phenotype
Abstract
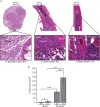
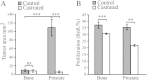
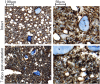
Prostate cancer generally metastasizes to bone, and most patients have tumor cells in their bone marrow already at diagnosis. Tumor cells at the metastatic site may therefore progress in parallel with those in the primary tumor. Androgen deprivation therapy is often the first-line treatment for clinically detectable prostate cancer bone metastases. Although the treatment is effective, most metastases progress to a castration-resistant and lethal state. To examine metastatic progression in the bone microenvironment, we implanted androgen-sensitive, androgen receptor-positive, and relatively slow-growing Dunning G (G) rat prostate tumor cells into the tibial bone marrow of fully immune-competent Copenhagen rats. We show that tumor establishment in the bone marrow was reduced compared with the prostate, and whereas androgen deprivation did not affect tumor establishment or growth in the bone, this was markedly reduced in the prostate. Moreover, we found that, with time, G tumor cells in the bone microenvironment progress to a more aggressive phenotype with increased growth rate, reduced androgen sensitivity, and increased metastatic capacity. Tumor cells in the bone marrow encounter lower androgen levels and a higher degree of hypoxia than at the primary site, which may cause high selective pressures and eventually contribute to the development of a new and highly aggressive tumor cell phenotype. It is therefore important to specifically study progression in bone metastases. This tumor model could be used to increase our understanding of how tumor cells adapt in the bone microenvironment and may subsequently improve therapy strategies for prostate metastases in bone.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

