Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Aβ generation in Alzheimer's disease
- PMID: 26993139
- PMCID: PMC4947125
- DOI: 10.1007/s00401-016-1558-9
Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Aβ generation in Alzheimer's disease
Abstract
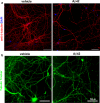
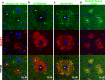
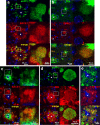
Alzheimer's disease (AD) is characterized by amyloid plaques composed of the β-amyloid (Aβ) peptide surrounded by swollen presynaptic dystrophic neurites consisting of dysfunctional axons and terminals that accumulate the β-site amyloid precursor protein (APP) cleaving enzyme (BACE1) required for Aβ generation. The cellular and molecular mechanisms that govern presynaptic dystrophic neurite formation are unclear, and elucidating these processes may lead to novel AD therapeutic strategies. Previous studies suggest Aβ may disrupt microtubules, which we hypothesize have a critical role in the development of presynaptic dystrophies. To investigate this further, here we have assessed the effects of Aβ, particularly neurotoxic Aβ42, on microtubules during the formation of presynaptic dystrophic neurites in vitro and in vivo. Live-cell imaging of primary neurons revealed that exposure to Aβ42 oligomers caused varicose and beaded neurites with extensive microtubule disruption, and inhibited anterograde and retrograde trafficking. In brain sections from AD patients and the 5XFAD transgenic mouse model of amyloid pathology, dystrophic neurite halos with BACE1 elevation around amyloid plaques exhibited aberrant tubulin accumulations or voids. At the ultrastructural level, peri-plaque dystrophies were strikingly devoid of microtubules and replete with multi-lamellar vesicles resembling autophagic intermediates. Proteins of the microtubule motors, kinesin and dynein, and other neuronal proteins were aberrantly localized in peri-plaque dystrophies. Inactive pro-cathepsin D also accumulated in peri-plaque dystrophies, indicating reduced lysosomal function. Most importantly, BACE1 accumulation in peri-plaque dystrophies caused increased BACE1 cleavage of APP and Aβ generation. Our study supports the hypothesis that Aβ induces microtubule disruption in presynaptic dystrophic neurites that surround plaques, thus impairing axonal transport and leading to accumulation of BACE1 and exacerbation of amyloid pathology in AD.
Keywords: 5XFAD mice; Alzheimer’s disease; Amyloid; Axonal transport; Aβ; BACE1; Cathepsin D; Dystrophic neurite; Microtubule; Tubulin.
Figures






References
-
- Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, Schliebs R. Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer’s disease beta-secretase (BACE-1) J Neural Transm. 2004;111:523–536. doi: 10.1007/s00702-003-0095-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

