Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases
- PMID: 26993512
- PMCID: PMC4844641
- DOI: 10.1007/s10571-015-0273-8
Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases
Abstract
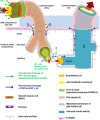
The lymphatic clearance pathways of the brain are different compared to the other organs of the body and have been the subject of heated debates. Drainage of brain extracellular fluids, particularly interstitial fluid (ISF) and cerebrospinal fluid (CSF), is not only important for volume regulation, but also for removal of waste products such as amyloid beta (Aβ). CSF plays a special role in clinical medicine, as it is available for analysis of biomarkers for Alzheimer's disease. Despite the lack of a complete anatomical and physiological picture of the communications between the subarachnoid space (SAS) and the brain parenchyma, it is often assumed that Aβ is cleared from the cerebral ISF into the CSF. Recent work suggests that clearance of the brain mainly occurs during sleep, with a specific role for peri- and para-vascular spaces as drainage pathways from the brain parenchyma. However, the direction of flow, the anatomical structures involved and the driving forces remain elusive, with partially conflicting data in literature. The presence of Aβ in the glia limitans in Alzheimer's disease suggests a direct communication of ISF with CSF. Nonetheless, there is also the well-described pathology of cerebral amyloid angiopathy associated with the failure of perivascular drainage of Aβ. Herein, we review the role of the vasculature and the impact of vascular pathology on the peri- and para-vascular clearance pathways of the brain. The different views on the possible routes for ISF drainage of the brain are discussed in the context of pathological significance.
Keywords: Cerebrospinal fluid; Cerebrovascular basement membranes; Interstitial fluid; Paravascular; Perivascular.
Conflict of interest statement
The authors, Erik N.T.P. Bakker, Brian J. Bacskai, Michal Arbel-Ornath, Roxana Aldea, Beatrice Bedussi, Alan WJ Morris, Roy O Weller, Roxana O Carare, declare no conflict of interest for this manuscript.
Figures

References
-
- Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45(4):545–552 - PubMed
-
- Abbott NJ (2013) Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36(3):437–449 - PubMed
-
- Alcolado R, Weller R, Parrish E, Garrod D (1988) The cranial arachnoid and pia mater in man: anatomical and ultrastructural observations. Neuropathol Appl Neurobiol 14(1):1–17 - PubMed
-
- Andres K, Von Düring M, Muszynski K, Schmidt R (1987) Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol 175(3):289–301 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

